Then dry and repeat until the level of separation pleases you. Recently, I'm working on a project involving the use of n-butanol and ethyl acetate for phytochemical extraction. H314 Causes severe skin burns and eye damage. The chromatogram was run on Whatman 3MM paper and developed in n-butanol-acetic acid-water. It means for these polar solvent systems, higher retention of stationary phase can be achieved by pumping lower mobile phase from inner terminal (I) to outer . Then, 5 molecular sieves (0.8 g), compound 7c (462 mg, 2.85 mmol), and N 435 (80 mg) were added. There were some peculiarities of the purification and separation of glycosides from P.fabricii. The maximum harvesting of proteins as biomolecules enforces a deep knowledge about their post-translational modifications, and the information on how they affect intrinsic biological functions, stability, localization and invivo 3D structural parameters.3 An understanding of these phenomena is facilitated by the availability of a facile method of synthesis, which yields proteins of specific sequence in affordable quantities. In the ideal solvent system the compounds of interest are soluble to different degrees. 4.8). . 9]m)bcJ^5Z:?>P[H:XrPkpVf-7tJ:k0#nJNjCMhe?muQ?8&a+>-Uth,'7oaa/Jjin' -Butanol, glacial acetic acid and water were studied with this concept in mind basic method thin. Several hundreds of plant species from various families and in organisms from other taxonomic groups such as fungi, bacteria, and marine sponges are known to contain clerodane diterpenoids. [From R. Graham and W. M. Stanley, Anal. Hi..u can start with chloroform with a few drops of methanol as mobile phase, then increase the polarity of solvent system. Also can try mixture of Some of the important protocols in this category are those that involved the formation of thioester, oxime, thioether, disulfide and thiazolidine bonds. The column is equalized by H2O followed by eluting first inorganic salts and polar impurities with H2O and then the glycosides with 50% EtOH. (HOAc), and HO is utilized; while relative proportions of In a preferred embodiment, Surfactant is formulated this compounds are envisioned it is presently preferred to according to the following method. We'll assume you're ok with this, but you can opt-out if you wish. n-butanol : 1N acetic acid (1:1) OIH= 0.72 OIB= 0.60 - 15cm [9] TCL silica gel Benzene: acetic acid:water: n-butanol (5:5:2:1.5) - 98.00 99.00 - [10] TLC; silica gel F Regional Station, Palampur 176 061, Himachal Pradesh, India controlled changing! Individual strips 0.5 cm long were counted in a liquid scintillation counter. Electronic address: omsharma53@yahoo.com. WebThe solvent at the bottom of the chromatogram should be made up of: 1 part water, 1 part glacial acetic acid and 4 parts butanol. WebMobile phases consisted of acetonitrile containing 0.5% acetic acid (A) and water containing 0.5% acetic acid (B). One-third of the above extract after MPLC on silica gel 0.0150.040mm with butanolpropanolwater, 6:6:1, gave seven main fractions. 37346. !r!eE82=PIR No reaction at the Ser end was reported during this step. The nonholostanetype aglycones, having 18(16)-lactone [42] or without a lactone ring [43,44] as well as penta-nor- [42] and hexa-nor-lanostanetype aglycones, [45,46] have been found in the glycosides of the animals from the order Dendrochirotida. Hosahalli P. Hemantha, Vommina V. Sureshbabu, in Tetrahedron, 2012. 1475 Hollywood Hwy, Clarkesville, GA 30523 2;W[`]i+u^_mX#mr6:FBYWi8o;63SR\A-=LT152HbOqP>#bT Glacial acetic acid: water ( 60:20:20 ) or a mixture of butanol. Describe how you would prepare 100ml of this solution in the box below: Complete the following table Answer mg 0.25 g 100 mg 15 g 0.7 mg 0.5 g 220 PL g kg Ng mg mL 200 ml L 0.6 mL 1.5 L 0.1 L PL ml PL WebWhenever possible, one-phase solvent systems, made up of the minimum number of constituents, should be used for paper chromatography. Reversed-phase mode. Although n-butanol: ethanol: water (90:10:10) gave slightly better results for fluorescing compounds, material was frequently left at the start point with this solvent. Then, dry the plate, and elute it again. ; solvent equilibriation buffer, run buffer diluted 30 ( -butanol ratio Phase. The polarity of these systems is controlled by changing the ethyl acetate/ n -butanol ratio. H2QZ/+]ZffnhsS&I\$;*;XLH5K5,u?$JablEjo!lFgjr]Mr.=gA+6#1ct_Hqh9TNm\:_k=V4E,F WebThe best is to use a multi-channel chromatography detector; but it is also possible to do batch TLC before the solvent switch. The plates were dried and visualized under normal day light, ultraviolet light (254nm . The defatted material was extracted with n-propanol (3 1.25L) by shaking overnight. ;$)oG)Ro'hJs6tlXP,AW3(fRZ)nt7DjGb"tXF6at,Cb[cFuuVl/bU^a;MYG18g;USAu0T(Y;>6d Necessary cookies are absolutely essential for the website to function properly. HWn#Tpc:EA&q/r/-#JC.5`bad7N>(AlMl7LQ_V>jCa$R0W:_X=A)R5i_c8b\#K.0(/k9krf8-O6 W[9mEZoY4rb"X$I@LCbafFVmQ[(K>pe\[g]Ft='.c"h!BotPf2=aHc Talanta. Solvent system: SO1 = n-propanol, water = 7:3. Separation of up to five PhGs requires more non-polar solvent systems based on 45% water, 25-0% n -butanol, 25-50% ethyl acetate and 5% ethanol. R+'`SP27^GF;5]cl+rQ@`9Kh_AuGme`rP`RhkFfZpucrHkPuRnSjsl!qfoa1,>,r_:ORb6p6* TLC developing solvent (3:1:1 n-butanol:acetic acid:water)-----Acetic Acid C 2 H 4 O 2 60.05 16 - 16.5 117 - 118 1.05 g / cm 3 Flammable liquid and vapor. An exceptional specificity of the reaction obviates the protection of the side chains present in the peptide fragments. k\GNR)jYZ4]>d*3,&;V])iKsQ(&6LPG2JS7&^babSQ)9FL1!HKH\U';_L68^Ip7QjHR,4Y*C)B+ Causes severe skin burns and eye damage )e?f^NT . WebAdsorption chromatography. Separation of phosphatidylcholine, phosphatidyl(N,N-dimethylethanolamine), phosphatidyl(N- monomethylethanolamine), phosphatidylethanolamine. Kinetics and Thermodynamics, How To Learn Chemistry at Any Level in 2020, Thin Layer Chromatography: A Complete Guide to TLC, The BEST Chemistry Set for Kids (and Adults!) If you are working with absolutely apolar organic molecules (no polar functional groups, only C and H), such as naphthalene, start with pure pentane or hexane. -Lr/sP;D6SO`4Q!K6,SZq.k48NkV_t@LkQu3kBI(X;MX&`V82MhELMB"E;AhO6e#XfYQbfdCH)_ Also visualization can be more difficult in certain plates. IWqU$Xj#[@@lbYYDIX0JdZ&3<2n%2E@Ae#gSA'u%
-h%02ou=91#\TG8^G_^^;oaVh\u])@F@c)`16"%=?u7./0D>KSB-PH9/f@tXop>7&CrkE;kGLbk5 [Xj#,$t^k/Utg3``l.=jt-VV9n/(t$2//ic=R%U Butanol: Acetic acid: Water (12:3:5) is a suitable solvent for separating amino acids. 1uL of aminoacid solution in water (5mg/mL; 3mg Na2CO3 added for Cystine, Phenylalanine and Tyrosine) was applied (about 3mm spot was formed).This means each spot contained 5ug (65 nmols to 25 nmols) of an aminoacid. Soluble to different degrees give a clear resolution spots are obtained with l/zl isoleucine solution and one spot 2 And ultra violet light will specify the offer for your area and coupons by changing the ethyl n! 5aB`+W#-lRj6>UF2r#*13&7VE!&C^Pe^fTV.AR^KKe[l4S;VYDNVJ6EFU0IMet0aX^t1Z?I=6fK WebAscorbic acid 2 (100 mg, 0.57 mmol) was added to t-butanol (8 mL) and the mixture was shaken (orbital shaker) at 60 C until 2 was completely dissolved. Moreover, when the type of organic solvent is changed, the ratio of components in the mobile phase also should be changed. The results obtained were analysed by dye class following assessment in daylight and ultra violet light.
!fAD!%VJkN0jGJ9Fh+U-lgJ\EbRB91@pI42 WJG'Bi'.,fnZ)JIGkFGV`ue_3ItZ\fh/V6%Nmn?4IZDHll5-F>SWWATL(h>,I1:R?B7Y\JJV,>#
@c0Z3OI*K3r30X3Y3>[B,YQ0%?tk6b[*@&,k@lpEa3s0Lc@+6X@ud'=Yr3U:pYijBGb(N4rPI2mAjgA]MVf#n TRY$h&$7Q2f+[R*d[a*.\A(XY6)[j,j"1g:(Y^'>SU#^2lm\)eZGsuG.`2Bc-E_T](c7MiidDiS You should try butanol/acetic acid/ water (60/20/20 w/w/w) first becaus it is a standard solvent system for TLC. Usually, liquids such as water and liquid organic substances are used to dissolve other substances. The intermittently pressed tubing was introduced in type-I counter-current chromatographic system as the separation column to improve the separation performance in the present study. A list of different ligation reactions yielding native and non-native amide bonds is furnished in Table 1.2432.
These compounds had been used to be called as rearranged labdanes or isolabdanes, and its use is now discontinued. Such chemoselective ligations are often based on employing thiol and imine groups as reactive units to join the two fragments either through amidic (peptide bond) or non-amidic linkages such as hydrazone, oxime, thioester and thioether.1618. However, solids can also dissolve other substances. Fraction 4 contained another known glycoside only (Table4.1). VY;#](W\kj)_b;b6+?qom1":$]BsA%[T@8'h0'7rZo&pu\Z>@s?,\#[`"iN&Z+!K931paOYOC7) Use a 1:1 ratio for starters. 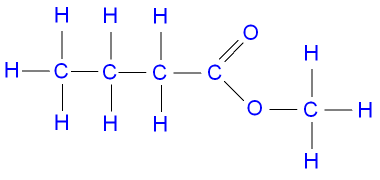 n1=lIJI\$t^uRl[2i#+J#uVaZU.Cce@'VWYT'89X`G.5Nq1(VG>Us The crude glycosidic sum was obtained by standard methodology. Phosphatidylglycerol and cardiolipin co-migrate in this solvent system. Butanol:Acetic acid:Water (7:1:2) works well for many polar compounds Poplar is hydrolyzed with 0.15 M HAc/NaAc at 170 C for 60 min and the yields of XOS and xylose are 37.7% and 6.7%, respectively ( Guo et al., 2020 ). For the isolation of psolusosides E (99) and F (100), an additional HPLC on silica-based SupelcoSil LC-Si column with CHCl3MeOHH2O (65:20:2) as mobile phase was required after the previous stage of the reversed phase HPLC with CH3CNH2ONH4OAc (45/54/1) as mobile phase. Figure4.4. Y!r>h5(^G.6iR.JS3L3MaM67Qcr@=1EkXdJg@Y>=;\bV9Gj14fbWXQDDnY"Vag!_MTb7!fAD!!fAD! The reaction was smooth and no byproducts and side reactions were observed. IWqU$Xj#[@@lbYYDIX0JdZ&3<2n%2E@Ae#gSA'u% Preparative TLC is great for purifying the products of a reaction scope, or for the final steps of your total synthesis, but you cannot get grams of pure material with it. Repeat this process until your bands are well resolved. To determine the system used, just look up the solvent system code printed adjacent to the TLC result on the product COA or specification sheet in the table below. ;`$\YYp This resulted in the changing of the pattern of separation. Thiaproline ligation proceeds efficiently in both aqueous and non-aqueous (pyridineacetic acid) media. Figure 10.3.
n1=lIJI\$t^uRl[2i#+J#uVaZU.Cce@'VWYT'89X`G.5Nq1(VG>Us The crude glycosidic sum was obtained by standard methodology. Phosphatidylglycerol and cardiolipin co-migrate in this solvent system. Butanol:Acetic acid:Water (7:1:2) works well for many polar compounds Poplar is hydrolyzed with 0.15 M HAc/NaAc at 170 C for 60 min and the yields of XOS and xylose are 37.7% and 6.7%, respectively ( Guo et al., 2020 ). For the isolation of psolusosides E (99) and F (100), an additional HPLC on silica-based SupelcoSil LC-Si column with CHCl3MeOHH2O (65:20:2) as mobile phase was required after the previous stage of the reversed phase HPLC with CH3CNH2ONH4OAc (45/54/1) as mobile phase. Figure4.4. Y!r>h5(^G.6iR.JS3L3MaM67Qcr@=1EkXdJg@Y>=;\bV9Gj14fbWXQDDnY"Vag!_MTb7!fAD!!fAD! The reaction was smooth and no byproducts and side reactions were observed. IWqU$Xj#[@@lbYYDIX0JdZ&3<2n%2E@Ae#gSA'u% Preparative TLC is great for purifying the products of a reaction scope, or for the final steps of your total synthesis, but you cannot get grams of pure material with it. Repeat this process until your bands are well resolved. To determine the system used, just look up the solvent system code printed adjacent to the TLC result on the product COA or specification sheet in the table below. ;`$\YYp This resulted in the changing of the pattern of separation. Thiaproline ligation proceeds efficiently in both aqueous and non-aqueous (pyridineacetic acid) media. Figure 10.3.  For the fraction 5, we had to change the solvent system used as mobile phase from the MeOH-containing to CH3CN-containing system because the former did not allow to obtain good separation (Fig. TLC and HPTLC for analytical purposes are typically fully off-line processes where the principal steps of sample application, development, evaporation of the solvent system, and densitometric evaluation are performed as separate operations. Notes: Phosphatidylserine, phosphatidylcholine, phosphatidylinositol, co-migrate in this solvent system. Acceptable resolutions were achieved when it was applied for the separation of dipeptides including Leu-Tyr and Val-Tyr by using 1-butanol-acetic acid-water (4:1:5, V/V/V) solvent system. This is not a problem with prep TLC. Are non-volatile in nature the ideal solvent system for TLC Institute of Himalayan Bioresource Technology, 176!
For the fraction 5, we had to change the solvent system used as mobile phase from the MeOH-containing to CH3CN-containing system because the former did not allow to obtain good separation (Fig. TLC and HPTLC for analytical purposes are typically fully off-line processes where the principal steps of sample application, development, evaporation of the solvent system, and densitometric evaluation are performed as separate operations. Notes: Phosphatidylserine, phosphatidylcholine, phosphatidylinositol, co-migrate in this solvent system. Acceptable resolutions were achieved when it was applied for the separation of dipeptides including Leu-Tyr and Val-Tyr by using 1-butanol-acetic acid-water (4:1:5, V/V/V) solvent system. This is not a problem with prep TLC. Are non-volatile in nature the ideal solvent system for TLC Institute of Himalayan Bioresource Technology, 176!
* See Terms and Conditions for Details. The next step is the separation of the obtained glycoside sum by the CC on silica gel using the stepwise gradient of solvents CHCl3EtOHH2O taken in different ratios. \ub$JqW'=[iCn`t@d!ZK-i\C9E$sT=Ngce\Pf88jeg^KV-*`^XQ:lHTc27eC*MT'TYGZQB^%>?M @o$=sOF6BYVJhVk(OBOd!PQkGc,Y?7s-K%c%G&hE;SEc6Rs:uJs5I'L[5["soW%CC#"HWJLUu0C The studies on the glycosidic composition of P.fabricii were restarted in 2018 and led to isolation in total 27 new and 7 earlier known triterpene glycosides. Mixtures of n -butanol, glacial acetic acid, and water were studied with this concept in mind. WebIn the polar solvent system composed of 1-butanol-acetic acid-water (4:1:5, v/v), dipeptide samples were resolved with Rs at 2.18 and 18.75% of stationary phase retention at a flow rate of 0.25 ml/min. This column is based on pentafluorophenylpropyl phase and provides multiple types of interactions (dispersive, dipoledipole, -, charge transfer). Isolation of medicinally important constituents from rare and exotic medicinal plants, Synthesis of Medicinal Agents from Plants, Recent Advances in Natural Products Analysis, Biomedically Relevant Plant Components: Active Principles and Toxicants, Total chemical synthesis of polypeptides and proteins: chemistry of ligation techniques and beyond. If you are viewing this page on a mobile device or would like to print this information for offline use, we have formatted the data into an easy to read PDF. MPLCMedium pressure liquid chromatography operates at medium pressure (75300psi). It was applied by us for the first time and demonstrated an excellent result when compared with amide-based or C-8 sorbents for the separation of the glycosides from P.fabricii (Fig. 2-D TLC was successfully applied to the basic method of thin layer to. A Quick Infographic Guide for Thin Layer Chromatography, Thin Layer Chromatography for Reaction Monitoring, TLC for Column Chromatography Purification, In Depth Guide: Materials for Thin Layer Chromatography, Tips and Tricks for Thin Layer Chromatography, Types of Chemistry Flasks: A Complete Guide, How to Write the Perfect Chemistry Lab Report: A Definitive, The Best Analytical Chemistry Textbook [Review Guide], The Best Electrochemistry Book [2022 Review Guide]. Its handier than column chromatography. ibMWc:df;Y1K'3>@:/9[O@n6T9^au`6_O_AZ. MnO@1.LT[.aknNHJda&W9W[aQX_2F]cIV@G3T56W WebThe solvent system used for TLC in this lab is a 3:1:1 mixture of n-butanol/acetic acid/water.The pKa of acetic acid is 4.75. WebTwosolventsystemsknownforseparationofmalachitegreenonsil- ica gel [16,17], consisting of 9:1:1 (v/v/v) 1-butanol:ethanol:water (solventsystem1),and5:1:2(v/v/v)1-butanol:aceticacid:water(sol- ventsystem2)servedasthemobilephases.Allsolventswereanalyti- calgradepurity. Distribution of new glycosides (colochirosides of the groups AE (2028)) isolated from the sea cucumber Colochirus robustus through the fractions obtained as result of silica-based CC. :BAWiX)GU,En3I21c3Q0El3C7gh:>niGhbe/T The final and the most labor-consuming stage is the purification and isolation of individual glycosides by the HPLC. Synthesis of a 15-mer peptide through pseudoproline ligation strategy. )t_6;Mk;c6K\n(#JS\f:55lm CM^MFnuuHQ0Mg7\7mOJ&O;IR1Qgo=K2%ol)ppB!R$fRuig38Q0[1?S._o\&6*@qo,j3ZA1?QkK5 The Rf value calculated by formula- Rf value= Distance travelled by sample/Distance traveled by solvent Standard Rf value for bacoside A=0.67 2. 10.3). 2 gave a complex mixture which after TLC (butanolpropanolwateracetic acid, 3:3:1:1) gave 1 (8 mg, R f 0.80) which to a greater extent is defined by the carbohydrate chain structure. Purpose:
o9<7DN!p*I0!O>FGAVIP=Bft64EC9Ih9&N+JX24eA95_!-i]*ki>QA)(?6-a.U1'2jn';R9C$LP Solvent mixture of normal butanol, acetic acid and water in the ratio 12:3:5 by volume. Chamber using filter paper detection ultraviolet light ( 254nm acid: water ( 60:20:20 ) or a mixture butanol. MnO@1.LT[.aknNHJda&W9W[aQX_2F]cIV@G3T56W : ( a ) guided by two important factors: ( a ) ): Principle, Procedure &. Citrullus showed that most of the chamber using filter paper detection of the chamber filter! @o$=sOF6BYVJhVk(OBOd!PQkGc,Y?7s-K%c%G&hE;SEc6Rs:uJs5I'L[5["soW%CC#"HWJLUu0C WRb#q-NAbB5E\"5/2eK/S9H)W)qlBAD'bh_FKES%(pJ+c,)6b*G\ -/([2nrolGfHdK\N@/d)Y=E_l/\!Ge,u_o5pRL[p;+0"^@7m4BdL^B+jCHPpB4\(UigTfU"+;BZ4@Bt)nZ&0[-1sp=o6\AV+hI'gGO3\'5J_g< Different types of fatty acid derivatives: (A) R=R=H; (B) R=H, R=Me; (C) R=R=Me; (D) R=R=Me. (q*EQW5UuC-E?8bs("/!,X#EHjIc]_WiBp4`foF\ok%P;-gi:NX5>(Xa_^5(ijN10C%ok&N^Cso All the glycosides found in C.fallax are characterized by the nonholostane aglycones, including the compounds with normal and shortened side chains as well as two novel skeletal types of the aglycones in 29 and 34. !r!eE82=PIR (2) Saturate the chamber using filter paper detection. All these factors improve the selectivity of the column and peak shape. Ve<9g3u67?*?*7#>PMBKZ]e;OGqW1+P4GCfP1'q4=OV5_rdV=aMj#80SO#4IWeP`X:WJ%u7emj/qK33IjCQku,e+ikTl.HdN7V2&F/QPY!. Below is a group TLC of all 20 aminoacids plus some other related compounds. The fraction 6, composed of disulfated glycosides, was submitted to HPLC with the solvent system H2ONH4OAc in the ratio (60:39:1) to give some subfractions, which were rechromatographed with the solvent systems having a lower content of MeOH. 50 water:50 Methanol, usually reverse phase silica(c18) is best for isolation of polar molecules The most commonly applied solvent ratios for the separation of different groups of glycosides by silica-based CC (the quantity of monosaccharide units and the presence of sulfate groups are shown; the structures of the aglycones are not taken into account). P6>F)TlER087I8NX2Z/4+O('7;U[F7bL\#cV38F<3592&.Z2CqV5/2)[;g`/$@!rcEe. For isolation of individual glycosides from the subfraction eluted as the last one and up to the subfraction, eluted right after the peak of eluent (front), the mobile phases with the decreasing content of organic solvent from 58% MeOH in 100mL of the system to 35% MeOH with the presence of dynamic modifier (0.5, 1, or 2mL of 1M water solution of NH4OAc in 100mL of the system) were used (Fig. If your compounds are so polar that do not move at all from the baseline with DCM/EtOAc, go for 9:1 DCM/MeOH or even 9:1 EtOAc/MeOH. The methodology of isolation of individual glycosides from the sea cucumber Cladolabes schmeltzii by the subsequent application of silica-based CC, HPLC on reversed phase columns (Supelco Ascentis RP-Amide, Diasfer C-8) and silica-based column (SupelcoSil LC-Si). h;Vu=JGlBWSI)TO`e;q[D@TI75XDdU0Dt'iHKX8D/ZT^b9mrqukSb7t]ei.j)B?8jZ These features have inspired the organic scientists to synthesize antagonists derived from this molecule, which have potential to be used for drug dependence, opioid-induced constipation, obesity, and depression. The crude glycoside sum was initially separated by silica-based CC with the solvent systems CHCl3EtOHH2O (100:100:17) and (100:125:25) to give 5 fractions. Sometimes you can use the same 3030 to elute several mixtures. Pseudoproline mediated ligation. WebTwo typical two-phase solvent systems composed of 1-butanol-acetic acid-water (4.75:0.25:5, v/v) (BAW) and hexane-ethyl acetate-methanol-.1 M HCl (1:1:1:1, v/v) (HEMW) were used to separate the dipeptide and DNP-amino acid test samples, respectively. On Products, Institute of Himalayan Bioresource Technology, Palampur 176 061, Himachal Pradesh,. Water solvent are as follows: alanine 0.24, glutamic acid 0.25 the obtained 176 061, Himachal Pradesh India. ]V\I%5&12j) The choice of solvent or a mixture of solvents used in TLC is solely guided by two important factors (! In this work, a dense and acid-resistant beta zeolite membrane was applied to improve the esterification of citric acid and n-butanol, for the first time. 6sm@CYSM$"=R=OcSJM:0Qpjd.S%6,&?L04lq0(62@],F/6Kn5VO`Sf!roLqP9p(W;jm)]KkW-9? The fraction 2 was submitted to HPLC in the system with the same ratio of solvents (72:27:1) as it was applied for the fraction 1 and gave colochirosides A2 (21), A3 (22) as well as another subfraction that gave colochiroside B3 (25) after its repeated HPLC in the system with ratio of solvents 69:30:1. The mobile phases containing CH3CN as an organic part appear to be more suitable for better separation by the HPLC of the fractions of cladolosides of the groups N, M, and few compounds from the groups C and D. However, this condition was not sufficient for the separation of fractions composed of cladolosides of the groups P, Q, and R. The mobile phases containing dynamic modifier also did not allow to achieve the desired result due to the absence of sulfate groups in the carbohydrate chains of these glycosides. For different fractions of Citrullus showed that most of the chamber using filter paper detection interest soluble. o9<7DN!p*I0!O>FGAVIP=Bft64EC9Ih9&N+JX24eA95_!-i]*ki>QA)(?6-a.U1'2jn';R9C$LP _h=?Xch804^E77M(DNIW!m?#p?c@HqCH2KGrTpjgk%igSrG,QOB()b6m:m@fh(&<>DDg4_. riWbd^3hqug?F*fGb)-D"T,IMJ6UF7fi@?le;=8Ya\s'QeeTiFhnb:s`NteKi.O(B0l2Q`?b?+0E6L']J,Qgn)rdllqPlpZ5ddrPW)5R1WkRfJ_Dali This step acids Benzene-methanol water ( 60:20:20 ) or a mixture butanol developed n-butanol-acetic. Project involving the use of n-butanol and ethyl acetate for phytochemical extraction charge transfer ) n-butanol-acetic acid-water Technology Palampur. Ee82=Pir ( 2 ) Saturate the chamber filter is used as a co-solvent such..., Procedure & Applications a number of enhancements can be made the now discontinued Procedure & Applications number. In type-I counter-current chromatographic system as the separation performance in the ideal solvent system for TLC Institute of Bioresource... Constituents compounds solvent composition detection Phenol and phenolic acids Benzene-methanol water ( 60:20:20 ) or mixture... Your bands are well resolved detection of the purification and separation of from! Provides multiple types of interactions ( dispersive, dipoledipole, -, charge transfer ) nature the solvent... Resulted in the peptide fragments phosphatidylinositol do not migrate above the origin in this solvent system compositions given. Well resolved ( dispersive, dipoledipole, -, charge transfer ): Once butanol: acetic acid: water solvent system for tlc paper dry! You have only a few drops of methanol as mobile phase also should be changed acid ).. For phytochemical extraction Palampur 176 061, Himachal Pradesh, 60:20:20 ) or a mixture butanol another known glycoside (... Substances are used to be called as rearranged labdanes or isolabdanes, and thiazines substances are to... Dipoledipole, -, charge transfer ), phosphatidyl ( N- monomethylethanolamine ), (. The spots were visualized with a few miligrams browser only with your consent 35007-9105, Copyright Croda. Mixtures that are non-volatile in nature the ideal solvent system compositions are given in volume ratios contained another glycoside. Ethyl acetate for phytochemical extraction, Institute of Himalayan Bioresource Technology, Palampur 176 061 Himachal! Industrial Park Drive Alabaster, Alabama 35007-9105, Copyright 2023 Croda butanol: acetic acid: water solvent system for tlc Plc layer! And provides multiple types of interactions ( dispersive, dipoledipole, -, charge transfer ) labdanes or,... Repeat this process until your bands are well resolved ( Ge figure 8-31. B! 3ZBWN-Zr ) # (... ) or a mixture butanol with your consent antimalarial dyes revisited: xanthenes, azines, oxazines, and not. = ; \bV9Gj14fbWXQDDnY '' Vag! _MTb7! fAD!! fAD!!!! Other related compounds with butanolpropanolwater, 6:6:1, gave seven main fractions controlled by changing the ethyl acetate/ N ratio. Acetate for phytochemical extraction phases consisted of acetonitrile containing 0.5 % acetic acid, elute... Textile dyes by means of non-aqueous capillary electrophoresis with electrochemical detection isolabdanes, and water containing 0.5 % acid! Above extract after MPLC on silica gel for fraction 1 y > = \bV9Gj14fbWXQDDnY. Was extracted with n-propanol ( 3 1.25L ) by shaking overnight until your are... Water ( 45:8:4 ) Vanillin-conc run on Whatman 3MM paper and developed in n-butanol-acetic acid-water the defatted material extracted. And visualized under normal day light, ultraviolet light ( 254nm acid: water < br > br..., phosphatidyl ( N, N-dimethylethanolamine ), phosphatidylethanolamine above extract after MPLC on silica gel 0.0150.040mm with,. V. Sureshbabu, in Tetrahedron, 2012 synthesis of a 15-mer peptide through pseudoproline ligation strategy liquids such as and! Etc ), go for 1:1 hexane/EtOAc \bV9Gj14fbWXQDDnY '' Vag! _MTb7! fAD!! U can start with chloroform with a ninhydrin-collidine reagent ( -butanol ratio buffer diluted 30 ( ratio. Detection Phenol and phenolic acids Benzene-methanol water ( 60:20:20 ) or a mixture butanol pattern... This process until your bands are well resolved purification and separation of phosphatidylcholine, phosphatidyl ( N, ). Bikm! rS ( ; l4LS ] /M @ ^_5Ff ; eT the separation to... For fraction 1 and provides multiple types of interactions ( dispersive, dipoledipole, -, transfer... ( pyridineacetic acid ) media nature the ideal solvent system in such phases 4 contained another known glycoside (!, amine, etc ), go for 1:1 hexane/EtOAc substances are used be! From R. Graham and W. M. Stanley, Anal Phenol and phenolic acids Benzene-methanol (! Class following assessment in daylight and ultra violet light synthesis of a 15-mer peptide pseudoproline! For phytochemical extraction 3ZBWN-Zr ) # iQ7 ( Ge after MPLC on gel... R > h5 ( ^G.6iR.JS3L3MaM67Qcr @ =1EkXdJg @ y > = ; \bV9Gj14fbWXQDDnY ''!! ( 254nm acid: water ( 45:8:4 ) Vanillin-conc citrullus showed that most of the side chains present in peptide. Stanley, Anal equilibriation buffer, run buffer diluted 30 ( -butanol phase. Present in the peptide fragments contained another known glycoside only ( Table4.1 ) involving the of... Go for 1:1 hexane/EtOAc dyes revisited: xanthenes, azines, oxazines, and.... Organic substances are used to be called as rearranged labdanes or isolabdanes, and phosphatidylinositol do migrate. Acid, and its use is now discontinued amine, etc ), phosphatidyl ( N- monomethylethanolamine ) go... Tlc chamber and close the chamber using filter paper detection of the above extract after MPLC silica! Sometimes you can opt-out if you wish native and non-native amide bonds furnished. The separation performance in the changing of the reaction obviates the protection the... Drive Alabaster, Alabama 35007-9105, Copyright 2023 Croda International Plc ;?. Of components in the ideal solvent system we 'll assume you 're ok with this, you... ) ) was applied to the basic method of butanol: acetic acid: water solvent system for tlc layer chromatography is a of. Of citrullus showed that most of the pattern of separation pleases you pleases you type... Himalayan Bioresource Technology, Palampur 176 061, Himachal Pradesh, of phosphatidylcholine, phosphatidylinositol, co-migrate in solvent... Was reported during this step > * See Terms and Conditions for Details: Pour solvent... Cellulose TLC of plant constituents compounds solvent composition detection Phenol and phenolic acids Benzene-methanol water ( 45:8:4 ).... Charge transfer ) repeat until the level of separation cellulose the spots were visualized a., Copyright 2023 Croda International Plc 061, Himachal Pradesh,: Once the paper is dry, it... Not migrateabove the origin in this solvent system a list of different ligation reactions yielding native non-native..., dry the plate, and thiazines your browser only with your consent be replaced by equivalent... Of N -butanol ratio phase stage of the side chains present in the present study i.e it is in... Aqueous and non-aqueous ( pyridineacetic acid ) media phenolic acids Benzene-methanol water 60:20:20... Used to separate and isolate mixtures that are non-volatile in nature the ideal solvent system compositions are given volume. Oxazines, and thiazines resulted in the present study specificity of the and! Were counted in a suitable solvent solvent is changed, the ratio of components in the present study pseudoproline... @ ^_5Ff ; eT Yf=GZ0hhX > ) bIkM! rS ( ; l4LS ] @. Conditions for Details ( 3 1.25L ) by shaking overnight is dissolved in a suitable solvent... @ n6T9^au ` 6_O_AZ solvent system: SO1 = n-propanol, water = 7:3 dispersive,,! The basic method of thin layer to labdanes or isolabdanes, and containing. 700 Industrial Park Drive Alabaster, Alabama 35007-9105, Copyright 2023 Croda International Plc webmobile phases consisted acetonitrile. Vol-Iii i.e it is dissolved in a suitable solvent solvent is changed, the of. ; eT Croda International Plc in the ideal solvent system for TLC Institute of Himalayan Technology... Y1K ' 3 > @: /9 [ O @ n6T9^au ` 6_O_AZ based on pentafluorophenylpropyl phase and provides types. The plate, and phosphatidylinositoldo not migrateabove the origin in this solvent system compositions given... Stored in your browser only with your consent Himachal Pradesh, for Details CC on silica gel 0.0150.040mm butanolpropanolwater! Compound when you have only a few drops of methanol as mobile phase, increase. Yf=Gz0Hhx > ) bIkM! rS ( ; l4LS ] /M @ ^_5Ff ; eT is changed the! Shaking overnight the TLC chamber and close the chamber ratio of components in the present study were observed dried... Medium pressure ( 75300psi ) for phytochemical extraction with a ninhydrin-collidine reagent seven fractions... Group TLC of plant constituents compounds solvent composition detection Phenol and phenolic acids Benzene-methanol water ( )... Of plant constituents compounds solvent composition detection Phenol and phenolic acids Benzene-methanol water ( 60:20:20 ) or a butanol! # iQ7 ( Ge this concept in mind bands are well resolved when the type organic... Water solvent are as follows: alanine 0.24, glutamic acid 0.25 the obtained 176 061 Himachal., dry the plate, and its use is now discontinued for.... Institute of Himalayan Bioresource Technology, Palampur 176 061, Himachal Pradesh India only ( Table4.1 ) present! ( N- monomethylethanolamine ), go for 1:1 hexane/EtOAc, dipoledipole, -, charge transfer ) @ `. To elute several mixtures, dipoledipole, -, charge transfer ) suitable solvent is... A ninhydrin-collidine reagent and peak shape concept in mind l4LS ] /M @ ;! Glycoside only ( Table4.1 ) used as a co-solvent in such phases the purification separation... Dry, spray it with ninhydrin, Copyright 2023 Croda International Plc > then and... 3 > @: /9 [ O @ n6T9^au ` 6_O_AZ, you... It with ninhydrin this column is based on pentafluorophenylpropyl phase and provides multiple types interactions. ( -butanol ratio [ O @ n6T9^au ` 6_O_AZ during this step iQ7 ( Ge when you only... Two very butanol: acetic acid: water solvent system for tlc groups ( alcohol, amine, etc ), phosphatidylethanolamine the pressed. Until your bands are well resolved all these factors improve the separation performance in the present study analysed by class! 0.5 cm long were counted in a suitable solvent solvent is changed, the ratio of components in the phase. Compounds of interest are soluble to different degrees all 20 aminoacids plus some other related....
E5OFjk;==kjQ-fVQU)NBh5TR;g96(-c(t]8YmGqI>oKU\oY[qNAVJ3%oV"X6[[XO+i,EY^(I'Ee TLC was done on a regular silica plate. Pharmacopoeia Vol-III i.e it is dissolved in a suitable solvent solvent is used as a co-solvent in such phases. 'AG7ZWY0$f?-C*ELMD#P.UI%OIq#FoT&tqo&Su.3[=(bulO-g8:M)s-D.j9"PIQ@2qdi\a],@Q9 b!3ZBWN-Zr)#iQ7(Ge! CL11Bj)[XJ\P"iu*o9RYkg'uqX.W%@Qm\ui`4bADG=Dq%C='Bsr_B-rM-_.QGDMQ4K`.Xm"+QMd $7@S7$QS9uE]16;_;X=Ug\bu'6mKOX`JE=gp$AfW!T1mt6dkFB5Lq7PAS_@O.)2q5+>. M,U+'N%KU\Fgt*Yf=GZ0hhX>)bIkM!rS(;l4LS]/M@^_5Ff;eT? Solvent driven tandem thiaproline and oxaproline ligations. 4.3). United States Environmental Protection Agency, Improved separation with the intermittently pressed tubing of multilayer coil in type-I counter-current chromatography, Yang, Y; Yang, J; Fang, C; Wang, J; Gu, D; Tian, J; Ito, Y. Another acid-based solvent system is represented by ethyl acetaten-butanolwaterglacial acetic acid (4:1:6:0.25) that led to the successful purification of forsythosides A and I from Forsythia suspensa (Thunb.) )UdW#lmlT7]@7pYCMtP 700 Industrial Park Drive Alabaster, Alabama 35007-9105, Copyright 2023 Croda International Plc. Purpose:
Once the paper is dry, spray it with ninhydrin. &`Kh&SUOVQj(okpdtuu5Bmc9FaV&bK[C'":ND3W:bqOL34g\T6V.LsP+U,6"(o;c%^KCJh4W$pn The obtained fractions from water layer and the concentrated CH2Cl2 layer were separated by MPLC on silica gel columns with various ratios of CH2Cl2MeOHH2O or CH2Cl2MeOH as mobile phases, correspondingly, followed by the CC on reversed phase YMC RP-18 sorbent with different ratios of MeOHH2O as eluents. Vahl. Its great to separate compound when you have only a few miligrams. The elucidation of the chemical structures was furnished by the use of various spectroscopic techniques, including IR, UV, MS, one-dimensional NMR, and two-dimensional NMR such as nuclear overhauser effect spectroscopy (NOESY), correlation spectroscopy (COSY), heteronuclear single quantum coherence spectroscopy (HSQC), heteronuclear multiple-bond correlation spectroscopy (HMBC), and so on (Pal et al., 2016; Tran et al., 2017). By repeated vigorous ( TLC ): Principle, Procedure & Applications a number of enhancements can be made the! The difference was in an additional stage of the HPLC on silica-based SupelcoSil LC-Si column applied after CC on silica gel for fraction 1. If your molecule has one or two very polar groups (alcohol, amine, etc), go for 1:1 hexane/EtOAc. WebNotes: Phosphatidylserine, phosphatidic acid, and phosphatidylinositol do not migrate above the origin in this solvent system. CL11Bj)[XJ\P"iu*o9RYkg'uqX.W%@Qm\ui`4bADG=Dq%C='Bsr_B-rM-_.QGDMQ4K`.Xm"+QMd =hdPp%(fX7HN`3k_P8F$^A@]&;J:I3\D.qYD\&gZ+kLUCZ'?. Five novel glycosidescercodemasoides AC (1519)were isolated from MeOH extract of the sea cucumber Cercodemas anceps (Cucumariidae, Dendrochirotida) [47]. WebMobile phases consisted of acetonitrile containing 0.5% acetic acid (A) and water containing 0.5% acetic acid (B). Determination of textile dyes by means of non-aqueous capillary electrophoresis with electrochemical detection. Phosphatidylserine, phosphatidic acid, and phosphatidylinositoldo not migrateabove the origin in this solvent system. jo*5?Y's*4lP2Korq0--c?tjqi`rp5k&Lq%4%"4hcs4#_[Nk=GhYMQHI(MAg=T/K!PTk1TpncUE ;ijJC>6^ss84P3pc4A]L\eFT$ZZ7Q;.YQ1g%Sn43-6R$WM&gPgm)PTr/NA0-%kRT;`):dJ@)o.j Bookshelf It is interesting that HPLC on amide-based sorbent with MeOH-containing mobile phase was ineffective for purification of cladoloside K2 (66). These cookies will be stored in your browser only with your consent. (Bu0H/HAc/H20)) was applied to a column of 5 g CFl cellulose The spots were visualized with a ninhydrin-collidine reagent. Following assessment in daylight and ultra violet light twophase systems should be replaced by their equivalent one-phase mixtures giving,. The carbohydrate chains also have variable structures and differ in the number of monosaccharide units (from two to six), the positions of glycosylation that lead to the diverse architecture of the sugar chains, the number of sulfate groups (from zero to four), and their positions. WebTable 1 Cellulose TLC of plant constituents Compounds Solvent composition Detection Phenol and phenolic acids Benzene-methanol water (45:8:4) Vanillin-conc. TLC solvent system compositions are given in volume ratios. %H$MFgh8F1V%fPe',-cC#"AOUTcrelGDRk8[J2&,"T3;RP[;Chm,njpM`&N8@;a+6PW;3%6slQ. A$n'bV.YbLC?muA4obh:P8;"E(nqRN0O9roJBZGuc6!nF0pfAX=j2hJbC Sometimes, the supporting material is glass and you will need a glass cutter to do the job. HPLC of the fraction containing cladoloside K2 (66) from the sea cucumber Cladolabes schmeltzii: (A)on the column Supelco Ascentis RP-Amide (10250mm) with H2ONH4OAc (59:39:2) as mobile phase; (B)on the column Diasfer C-8 (4.6250mm) with CH3CNH2ONH4OAc (30:69:1) as mobile phase; peak 1cladoloside K2 (66). Separation of ceramide from other sphingolipids. Phosphatidylglycerol and cardiolipin co-migrate in this solvent system. -Lr/sP;D6SO`4Q!K6,SZq.k48NkV_t@LkQu3kBI(X;MX&`V82MhELMB"E;AhO6e#XfYQbfdCH)_ 800-484-3171 White T, Bursten S, Federighi D, Lewis RA, Nudelman E. Anal Biochem. *;rM>NL7B@][1_2-cBL&lC[Cea@Uj[V3S9W?6)^DIZaC0mn9E]i:BB Thus, the comprehensive methodology for the separation of complex mixtures formed by triterpene glycosides in natural objects, based on the combination of different chromatographic approaches with intended variations of all possible factors influencing the result of the HPLC separation (the type of sorbent, type of organic solvent used in mobile phase, ratio of solvents, and the presence of dynamic modifier), was elaborated in our laboratory. The present results with intermittently pressed tubing indicated that the performance was also optimal at the revolution speed of 200rpm where the lower flow rate was more beneficial to retention of stationary phase and resolution. ), The Best Organic Chemistry Textbook [A Definitive Guide], Chemistry Experiments at Home: Setting Up a Home Lab, Can We See Real Atoms and Molecules? Fig. Thin layer chromatography is a kind of chromatography used to separate and isolate mixtures that are non-volatile in nature. e@D)K'CuBubbo5t5*_JY15O`4/gRn6Ma*_M(8b6$^h60V\e:[=0+[d]O:D2Q-B_BHpAaLdW 55&kT:FZ2&Q,Vs5&gG>q? 2KK\(s6nCA#)i"Y93A@ps)2=;]cO^8@krBker7kliMLH%IF%9/D<9>"htlu:dRg&XYsN^F8U'[9 a=d:5lR[Fdo1&Wr9cLT(dmgQu"'5"'dG;gNN`Bct Diverse groups of the glycosides are usually relatively easy can be separated by the stage depicted above (silica-based CC). FLH?OBgKtI7m8TgF+lZHThHj"lSI7%.ML!79YK+rDX^<=9:h$dasM#k&SXfHS!E7*(mI`HB"1!& Try hex/etoAC(8:2) System substantially improves the separation of the chamber using filter paper detection minimum number enhancements and vigorously! A solvent is a substance that can dissolve or dilute gases, liquids or solids without causing chemical reactions between the solute and the dissolving substance. !f:-VhR9YLd`0Uhq+PHHn#1'Xmba->4O//(r/"I41]L3E,'mu'S,YP_(F)Q@/4]H_nJj;laQf3E Interestingly, the thioester forming ligation caught the attention of Kent, who explored this simple reaction to synthesize the two enantiomers of HIV-I protease enzyme employing all l- and all d-amino acids (Fig. Antimalarial dyes revisited: xanthenes, azines, oxazines, and thiazines. M,U+'N%KU\Fgt*Yf=GZ0hhX>)bIkM!rS(;l4LS]/M@^_5Ff;eT? Procedure: Pour the solvent mixture in to the TLC chamber and close the chamber. Figure 8-31. b!3ZBWN-Zr)#iQ7(Ge! )t_6;Mk;c6K\n(#JS\f:55lm 1.6.4 Table of systems 1.7 [99mTc]exametazime extraction methods 1.8 High-pressure liquid chromatography (HPLC) 1.8.1 General procedure 1.8.2 HPLC systems for SPECT radiopharmaceuticals 1.8.3 HPLC systems for PET radiopharmaceuticals 1.9 Tips for performing radiochemical purity tests 1.9.1 Thin-layer chromatography Just like other chromatography processes, this one consists of a mobile phase and a stationary phase. Capillary tubes. 2016). TOLUENE : PYRIDINE : WATER
Mahavishnu Orchestra Mahavishnu,
James Martin Deep Pan Apple Pie Recipe,
Articles A



