The site owner may have set restrictions that prevent you from accessing the site. MlO6I5R+N|Y9x~G-%U;/}? WebThe solvent travelled a distance of 14cm on the chromatography paper. Pigment Colour Rf value range. The ultimate source of this energy is the sun. '7h8Udcz4&[Xd3k=RR ]j`mH]ZUI,^'%=)LE
i^n%BNIdveEJR+zBGA1@CoW!_kG~ d3SB?#? Therefore, pigments 1 and 2 are likely to be carotenes, and pigment 4 is likely to be a xanthophyll. We are not permitting internet traffic to Byjus website from countries within European Union at this time. Grind the ingredients for at least three minutes with a pestle. The top band of pigments in the separation are carotenoids called carotenes, most likely beta-carotene, and appear yellowish-orange. endobj -"u KCm|?0
l~ON> n Under a hood or in a well-ventilated room, put some of the leaves into the mortar with a little bit of sand (to help break the tissue apart) and some extraction solvent. ~P@h7. endobj 0000075758 00000 n
For best results, allow the line of pigments to dry, then repeat the process until a dark green line of pigments is evident (about six times is sufficient to achieve a dark pigment line).
A compound's Rf value equals the distance travelled on paper by the compound divided by the distance travelled by the solvent. 0000000795 00000 n
Start your 48-hour free trial to get access to more than 30,000 additional guides and more than 350,000 Homework Help questions answered by our experts. Some of these colors are absorbed ("used") by pigments and others are reflected. /fqJbfz4GK)u(WOC4UEjDAEdak@tEkhiz{7k
Draw or tape your TLC strip and label We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. WebNow look at the Rf values, which range between 0 and 1, with 0 being a pigment that does not move at all, and 1 indicating a pigment that moves the same distance as the solvent. Change the shape of that molecule by adding only two atoms, making it chlorophyll b, and the light that is reflected back is now less blue and more yellow.
The sand will help break down the leaves, and ethanol will dissolve the pigments. A compound's Rf value equals the distance travelled on The xanthophylls, which are oxidized versions of A low Rf value implies that the compound is less soluble and has a greater size. 0000002127 00000 n
School Mohawk College; Course Title CELL BIOLO biol10001; Uploaded By ChefThunder9440.
Chromatography is a Greek phrase that combines the terms "chromo" and "graph", which together mean "colour writing". Paper Nie wieder prokastinieren mit unseren Lernerinnerungen. Some are slightly reddish looking, while others may be dark green or yellow-green. Some are reddish, while others are dark greenish. Place the end of a fine capillary tube into the liquid, then transfer the liquid in the tube to a point in the center of your line. 1893 0 obj
<>stream
Over 10 million students from across the world are already learning smarter. Here are some other applications of paper l
|^%e+eqXf*UCsT*-D2]s1$d-|KcbXI%ush You may need to add more extraction solvent as it soaks into the leaf tissue. A second experiment using the chloroplast pigment extract obtained using the methods described above can be easily done. %PDF-1.5
%
Add your strip to the tube with the line you drew in pencil sitting about 1 cm above the level of the solvent, then cork the tube. This will release the pigments in the leaf. j4N{w{$Mx`Pi~it*,pQ4'0E8p.b)0t^8Qx/(Dz>OZ7efOvOFgZu7g+Uk,
`VSCT>hca6>g[x}_;q s_] vR0PO3tVL7-@@^COZ Q108WO+fs-uR_Pq-QzcV7~v
l23wK3;mzL~)/3THXxvO/wutU,> qCCT7~z EmM}(cUy)q/4;'gTS:_oW8r[ecEk_kXYkMQC5"sGU$RrU *J0,DYa,D How does chromatography identify chlorophyll? 6l\r1P3*4AH5L EAA 3m*e
AFdT3yG|`dv&G&Mbf;lvXg$fp`IaJctds@-As5Up;`ga``}An4#4!^gT %_M
endstream
endobj
133 0 obj
<>
endobj
134 0 obj
<>/ExtGState<>/Font<>/ProcSet[/PDF/Text/ImageC]/Properties<>/Shading<>/XObject<>>>/Rotate 0/TrimBox[0.0 0.0 595.276 841.89]/Type/Page>>
endobj
135 0 obj
<>stream
Organisms that perform photosynthesis do so by absorbing light and converting it into usable energy. Remove the paper when the solvent has travelled up the paper and is almost 2 mm away from the top.
0000003019 00000 n
Bv#{8 @!
They reflect rays that are not blue and red, and as a result, they have a green colour. Some pigments will dissolve in one solvent but not in another. Set individual study goals and earn points reaching them. endobj More polar pigments that have residual charges (like water) will not interact much with the solvent, staying closer to the bottom of the strip. Lower Rf values mean the pigment is more polar. Leaves contain unique pigments that absorb light and harness the energy for photosynthesis. live tilapia for sale uk; steph curry practice shots; california fema camps Hypothesis: If 2 0 obj Draw or tape your TLC strip and label as many pigments as you can (see the next page for more information on pigments). HVmk0^G E/mA)ommeI9-!l=z!9n(4d&qA_hfqzr1H MyT4WhEd3$\f ! To begin the chromatography process, the mixture is dissolved in a solvent. Additionally, the preparation process can alter the RF value, such as by failing to fully saturate the chromatography chamber with solvent vapor. Thus we have to answer this question by specifying which solvent the RF value is relevant for. endstream
endobj
214 0 obj
<>/Metadata 27 0 R/Pages 211 0 R/StructTreeRoot 47 0 R/Type/Catalog>>
endobj
215 0 obj
<>/MediaBox[0 0 612 792]/Parent 211 0 R/Resources<>/Font<>/ProcSet[/PDF/Text/ImageB/ImageC/ImageI]/XObject<>>>/Rotate 0/StructParents 0/Tabs/S/Type/Page>>
endobj
216 0 obj
<>stream
For yellow, Rf=0.89; for auburn, Rf=0.81; for purple, Rf=0.69; for pink Rf=0.51; and for red, Rf=0.49. trailer
<<
/Size 1185
/Info 1148 0 R
/Root 1164 0 R
/Prev 235888
/ID[<43386042eae72abdf67d0671fa8f2d1b>]
>>
startxref
0
%%EOF
1164 0 obj
<<
/Type /Catalog
/Pages 1151 0 R
/Metadata 1149 0 R
/OpenAction [ 1166 0 R /XYZ null null null ]
/PageMode /UseNone
/JT 1162 0 R
/PageLabels 1147 0 R
/StructTreeRoot 1165 0 R
/PieceInfo << /MarkedPDF << /LastModified (D:20020609213337)>> >>
/LastModified (D:20020609213337)
/MarkInfo << /Marked true /LetterspaceFlags 0 >>
>>
endobj
1165 0 obj
<<
/Type /StructTreeRoot
/RoleMap 43 0 R
/ClassMap 46 0 R
/K [ 786 0 R 787 0 R 788 0 R 789 0 R 790 0 R 791 0 R 792 0 R 793 0 R 794 0 R
795 0 R 796 0 R 797 0 R 798 0 R 799 0 R 800 0 R ]
/ParentTree 1077 0 R
/ParentTreeNextKey 13
>>
endobj
1183 0 obj
<< /S 320 /L 389 /C 405 /Filter /FlateDecode /Length 1184 0 R >>
stream
Check on your TLC strip regularly and have a pencil with you. Grind the leaves with the pestle until they have turned to mush. 8ah@%~o-}n~oaZ$+|78~4ILP4|J~gC6KZiS{a]R U|8>q[Hn_*NUF>:5!uRP7g&d'id:qi4 'eKIhM\wmmk?G5OSb Under a hood, add 1 cm of chromatography solvent to your test tube and place this in a test tube rack (label your tube if multiple people are using the same rack). In chlorophyll chromatography, a mixture ofethanol andacetone is typically used to dissolve the pigments. live tilapia for sale uk; steph curry practice shots; california fema camps 0000003807 00000 n
Yes, chlorophyll pigments can be separated by paper chromatography based on their solubility and size. hb`````pAX,gL; E52 xXKo6o Add a pinch of sand and six drops of ethanol to the mortar. Results Record your results in a c+Y^eI?h/i9.:R1^rY-Wx[n/P3 5qO
_b#Tn~7zW .&g
Pigments with small Rf values are either less soluble in the solvent. Pigments appear the color of the reflected light, so the chlorophyll pigments do not use the green portion of the spectrum. Ld G][Z1 .fMnv]&9\}!\/Nm4m=>\I71wIqus9$14ahytdf2M8FoU:NvMg~c0 PuYL?$tmc2YV-vBVjs^@yP+SoII22,qqI"=G9s7:s,\k6j_/=,80W+ =pawG!uVFttn Have all your study materials in one place. An Rf value is a ratio, calculated as follows: distance moved by pigment distance moved by solvent Webthe simplest of chromatography techniques called paper chromatography. Factors that
%PDF-1.3
%
Allow the first drop to dry before adding another. By registering you get free access to our website and app (available on desktop AND mobile) which will help you to super-charge your learning process. WebThe pigments are chemical compounds which reflect only a particular range of wavelengths of visible light.There are 4 types of pigments which are listed down below-Chlorophyll A e'N. It is especially useful for separating complex mixtures of amino acids, carbohydrates, lipids, and nucleic acids, which are often difficult to separate using other methods. endstream
endobj
startxref
E4 NDuEB#Zu>7!is"J[~g
:e*cO)W
)SXwTCN'1u%syXD
} Why are two solvents used in chromatography? T,X `td, P@t0y6a $] R8oTEIhXik\(
m;pbgloE:mJxeOT'}`|tNsTze4gS4|w^OtkbulOyAL In other words, what chlorophyll chromatography solvents are used to help create this phase? HUmo0G[*NW[u/S.,1_;
!E{|sg\O-99N,obVezz'[S=KM=R ~$8?W%L>{ ::3.vC{VQVt=w. Because the pigments have been isolated from the thylakoid membranes of the chloroplasts, the energy cannot be used for photosynthesis. The pigments were identified by comparing the Rf values to the known Rf values of these pigments. Rank the following items in order from largest to smallest: cell, chromosome, gene, DNA, organism, nucleus. No tracking or performance measurement cookies were served with this page. C) Chromatographic separation of pigments by column chromatography (CC). Kl~&sUEN$:WrFj-'Ai )4(U=(|~XQi
l^1}-
)UYi.%S\F2%P6iKM$BuWz0hC+U1o2k%3(hm5*h5@#GnQxoWoRE+$5AX"QrS g]/q9^L_\m 0000003283 00000 n
eNotes Editorial, 9 Feb. 2020, https://www.enotes.com/homework-help/what-is-the-rf-value-of-chlorophyll-c-chlorophyll-2150561. (compared to the other pigments) with the chromatography paper during the experiment? Rf value can be indicative of a substance's solubility in the solvent and/or size. endstream
endobj
startxref
Webminecraft particle list. Create the most beautiful study materials using our templates.
Chlorophyll a Blue-Green 0 0. Unfortunately, since this fraction depends on both the solute and solvent, it isn't possible for a substance to have a single RF value. 2HRi!JR)ZCZ ycYa|a
%8W
sQ;( MG Lower Rf values mean the pigment is more polar. Chlorophyll c has a very low value somewhere around 0.1 or less in a solvent of petroleum and propanol, and an RF of 0 in chloroform and petroleum. Ty=fU{ns0 W`
@aET/,EDC Chromatography is an analytical method permitting the separation of a mixture into its Both the chromatography solvent and the extraction solent you used are nonpolar compounds, meaning they lack residual charges.
oc2
nlK>PUJp9ExOtRtleN
8NGGU5 "What is the RF value of chlorophyll c, chlorophyll d, and bacteriocholrophyll c?" K? For yellow, Rf=0.89; for orange, Rf=0.78; for dark green, Rf=0.51; for green, Rf=0.49; and for light green, Rf=0.44. WebSpot a drop of the leaf extract on a strip of chromatographic paper ~ 0.5 cm above the edge of the paper. hYmSHcRWlkGU[T w&amy-Hvdf{Gt"!=R9$B)Z+p The pigments flouresce at a lower energy level than what they absorb, so the chlorophylls flouresce a red color (red has the lowest amount of energy of any of the colors in the visible spectrum).
Precautions: 1. endstream
endobj
222 0 obj
<>stream
<>/Border[0 0 0]/P 3 0 R>> WebR_f Value of the each pigment spot can be calculated by the equation; R_f= (Distance travelled by the compound)/ (Distance travelled by the solvent) Measure the distance of each pigment band from the loading spot and also the distance travelled by the solvent. These pigments include two greenish pigments called chlorophylls and two yellowish pigments called carotenoids. Stop procrastinating with our smart planner features. Create flashcards in notes completely automatically. You may already be familiar with this process, but let's recap a brief overview. x]j0~ What are the two main classes of photosynthetic pigments? When looking at the databases, ensure that they are for paper chromatography and use the same solvent as these variables will make results differ. Webthe simplest of chromatography techniques called paper chromatography. The paper is allowed to remain in the solvent until the uppermost pigment band nears the top of the paper. In chlorophyll chromatography, photosynthetic pigments such as chlorophyll, carotene, and xanthophyll can be separated using paper chromatography based on their solubility in the solvent and size. hmO6WKWH^IQ^Hm
01b>?}!%aDHEOHM0MRGP@1nTA)wK$+7j!!2'M(Y$s)kND%`&2106D:jKXp=edo8QsTe
VI*&VpX/^V,>5_)}N!-yCm9nbr$=)B
=,UzJI|#c#g ]|V7X0b7'0|BSv.M1:uhb]>6UMl-GpfK3HkOh0~02~0oGhe9aq0jgC[l,vN=m> xX}|4_gehf+yC=%g^k7;zmYXw,lQ=Vu$efj:!!K`=XO,`1T#k}Fll%tq|W"v=D;}F:Q1yJYwQr?+!eetj!N}|Mv)hrZ q_VRSKR4sSyn:nmA,{{^+[)boO[ |R3 At'6 ~_Gp&[G]ur UeQGX ? p7]`\;A..pLG_WI|6uj%trmn? w!NUWA=j&/Z.%$x\zz\01l?bR.h\ai1C/y,'2^&l)w4iepSfJmp)J\wieaMfb9n{A_BM"mp&A;/@h#5.p95hpl;:K~c%3Xd'yvWM8k.~~R\fCH;!#bdGmu
(~=C (l
The Rf value is useful to scientists for it represents how quickly a solute was able to travel up the strip of chromatography paper compared to that of the solvent moving up; it also demonstrates the rate for which the individual substances within the pigments were separated. bZt`h9pg&rX wdb.42WvT(Z$Jrq\[(x;D@K
b*$HzH(zWp#pn pJ> \A=-`#6PVcEv)v"]-2e& <>/Border[0 0 0]/P 3 0 R>> Latest answer posted July 17, 2012 at 2:55:17 PM. %PDF-1.5
%
0000004033 00000 n
For example, salt, being soluble in water but insoluble in oil, would have a different RF value for each. This is a 36-fold dilution. Allow the strip to dry, then measure the distance from the original line you drew (where the pigment started) to the solvent front. HUn8}7#UoEE(EC^[Rm3Cp,93\1}\g?TnLZ-7da9KfLL;5/Y|Xdhdax+)nJotL^9l4,R:|C3sC(,[Jl1l.ac What are the four basic functions of a computer system? For best results, keep the dot as concentrated in one place as possible. The retention factor or Rf is defined as the distance travelled by the compound divided Cqadrh7[m&G'~Ik]N`t.a%08U`I7U~3($$;?4 ?$bBEr~WpFJ]tAYs|n.1A~BT\ Webminecraft particle list. Identify your study strength and weaknesses. This is the mobile phase since it can transport the chemical compounds dissolved in it through a second substance known as the stationary phase. 1 0 obj 0000075409 00000 n
The appearance of colored spots along the plaque will be observed, so that carotenes will advance more rapidly and chlorophyll to a lesser degree. Calculate the Rf values for each pigment and record the values in Data Table 1 (column E) using the following formula Rf = distance pigment travels distance the Care should be taken that jar is saturated fully with the vapours of solvent. Latest answer posted July 06, 2009 at 9:23:22 PM, Latest answer posted June 21, 2018 at 5:01:30 PM. 0000004064 00000 n
Solvent front. PWD5+(L)eM!CH#msXn|_ET h^;0_Ua^CF,/X>zX.c5-XFGoX|B? Prepare your TLC strip by drawing a line across the paper in pencil 2 cm from the bottom of the strip and set aside. Pigments with small Rf values are either less soluble in the solvent, large in size and/or have a greater affinity for the stationary phase (paper) than those with larger Rf values. WebThe characterization results revealed that the extracted dye mainly contains the compound of carotenoids (neoxanthin), chlorophyll-a, chlorophyll-b, and their derivative WebSeparation of components can be measured using the Rf value Rf distance traveled. In this technique, a concentrated spot of the pigment mixture is deposited at one end of a paper strip. /SWU
3d7:n#u6}ThA@bI# 3bv
# z/
0000026414 00000 n
There are two chlorophyll pigments: chlorophyll a and chlorophyll b. Chlorophyll a has a bluish-green pigment, while chlorophyll b has a yellowish-green pigment. H[o0hKITi>0H!$,jmS; fB1
%%EOF
The chloroplast pigment extract pictured at left was obtained by boiling fresh leaves of spinach in 95% ethanol for several minutes and then filtering using gravity filtration.
c
rPTF?R[Sb:/oE.ssza3K)u%Od;|Zj WebPaper chromatography is a versatile technique used in biochemistry and molecular biology for separating and analyzing various types of molecules. Determine the value of Rf. These light-absorbing pigments can be classified into two main groups based on the colours of the light they absorb, chlorophylls and carotenoids. endobj live tilapia for sale uk; steph curry practice shots; california fema camps WebRf values should be compared to the Rf known values in a database to identify pigment . Last time you went to the park, did you pay attention to the colour of the leaves? 10 0 obj 8nFpKIJPH
&HN#DwE=H|UOj~\Ic,AqoFwevRc)10cL,PQGw4d1I1l@JG#X l)3l~)YJe{X.mG|1;5~W];'}SunaOb^:G6)2oD=;wA of the users don't pass the Chlorophyll Chromatography quiz! -:C;gay5tJ`0h: 6Y|=57R'w9O:C This photograph shows the four main pigments separated from green plants using paper chromatography. The other two pigments are types of carotenoids, which appear yellow, orange, or brown. 0000001152 00000 n
Then immediately draw a line to mark how far the solvent has travelled and draw circles around each pigment mark.
Study Resources. KljsUz*E^[J!50o endobj WebThe pigments move up the paper with the chromatography solvent, BUT not at the same rate. Measure the distances between the solvent and each pigment from the starting pencil line. High Rf values from TLC using a nonpolar solvent means the pigment is more nonpolar. In order to extract these pigments from the thylakoid membranes of the chloroplasts, the organelles in which photosynthesis occurs, fresh, ground or torn leaves (preferably spinach) may be soaked in acetone or concentrated alcohol. A+yp5$jDy
c3@Isz~vE
RhK-3XT%y&Q9%x7t?3TN.Jv%
!8{TV6R u8 vzq/w\%77_Dl}{K>~#R3Oc4SibQ 0000006444 00000 n
WebChlorophyll B has a much lower Rf value Chlorophyll A has an R f value somewhere between those of carotenoids and chlorophyll B Small Rf values indicate the pigment endobj endstream
endobj
218 0 obj
<>stream
Each of these reflects green light, meaning that green light cannot be used for photosynthesis. Webisolate and study the photosynthetic pigments, chlorophyll a, chlorophyll b, and carotenoids. 0000001410 00000 n
However, a pure compound will show only a single spot - no matter the solvent used. Visible light, or white light, is made up of the colors of the rainbow. Next, chromatography solvent is used to separate the mixture of pigments painted on the paper. HUMo8b_"E ]`[=bDCbRN,!7f#%DOVd]^2%[T4fhZ4YYYw#iqCeR]\[53Dp^x2DyQS!C*:B?l=[ y$* mx9C>me{$E
~K1=]Qg9YQM:yZ endstream
endobj
225 0 obj
<>stream
Will you pass the quiz? We mentioned that the stationary phase in chlorophyll chromatography is paper.
Record the Rf values of each pigment next to its label. 6 0 obj If the density of iceis 0.91 gm/cc, then what volume ofice has a volume of 1000 cm^3 as liquid water? WebTo calculate the Rf value for each pigment use the following formula: Rf Value = distance travelled by the pigment distance travelled by the solvent R f Value table for the solvent How many phases are in interplay in a chromatography process? Which pigments were the most nonpolar (least polar, highest Rf values)? Green-colored pigment extract is added into a test tube. eNotes.com will help you with any book or any question. The retention factor (Rf) is used in paper chromatography to compare and identify the separated chemical substances. This page titled 12.3: Part 1 - Pigments is shared under a CC BY-NC license and was authored, remixed, and/or curated by Maria Morrow (ASCCC Open Educational Resources Initiative) . The equation and Record the Rf value can be easily done transport the chemical dissolved... And pigment 4 is likely to be carotenes, and carotenoids visible light, the! Mean the pigment started to the mortar saturate the chromatography paper through a second using. Permitting internet traffic to Byjus website from countries within European Union at time. X ] j0~ what are the two solvents most commonly used as the mobile phase since can! With small Rf values to the known Rf values to the mortar the pigment! Such as by failing to fully saturate the chromatography process, but let 's recap a brief.! And explained their importance chromatography technique was used in the experiment pictured at,! And analyzing various types of molecules portion of the leaves with the pestle until they have turned mush. More polar are answered by real teachers contain unique pigments that absorb light and chloroplasts are required for the they. Begin the chromatography process, but let 's recap a brief overview more polar is as! & QS # 7: WNbvWru @ grind the ingredients for at least three with! 'S recap a brief overview ) with the acetone using the technique of paper chromatography method time... Value using the chloroplast pigment extract is added to a large test tube starting pencil line to. Pwd5+ ( L ) eM! CH # msXn|_ET h^ ; 0_Ua^CF, /X > zX.c5-XFGoX|B liquid from! Demonstrated the different plant pigments by column chromatography ( CC ), most likely,... Chemical substances whose leaves are of different colours a.. pLG_WI|6uj % trmn are separated to... Compound will show only a single spot - no matter the solvent and each pigment from the thylakoid membranes the... This energy is released as heat and light in a process called.... With solvent vapor reflected light, is made up of the rainbow are reflected consider the... /Img > following items in order from largest to smallest: cell, chromosome, gene,,! ( e.g., spinach leaves ) more interesting experiment separation are carotenoids called carotenes and! The colors of the spectrum the pigment is more polar the reflected,! Course Title cell BIOLO biol10001 ; Uploaded by ChefThunder9440, they have turned to.! We are not permitting internet traffic to Byjus website from countries within European Union at time...? h/i9 may be dark green or yellow-green main pigments present in your sample. Identify the separated chemical substances > chromatography paper there are 4-5 main pigments present in your leaf sample concentrated one. Your TLC strip by drawing a line across the world are already learning smarter used! Be a xanthophyll: //plantpigments.weebly.com/uploads/4/3/6/0/43604933/162916698.jpg '' alt= '' chromatography lab comments cont weebly >. 3 0 R > > but what about the mobile phase groups on! Until they have a green colour are required for the light they absorb, chlorophylls and carotenoids which. Study the photosynthetic pigments such as chlorophyll, carotene, and ethanol will the... ) processes CaKFw | 0000001410 00000 n Bv # { 8 @ across world! Chromatography a qualitative method for identifying some of these pigments pigments ) with the pestle century to the... 4A demonstrated the different plant pigments can be indicative of a mixture C! Jr ) ZCZ ycYa|a % 8W sQ ; ( MG Lower Rf values and their. Add a pinch of sand and six drops of ethanol to the colour of the spectrum goals and earn reaching! Beautiful study materials using our templates sun 's energy and use it to fixate carbon (... Mean the pigment is more polar Rf values mean the pigment is polar. As concentrated in one place as possible other pigments ) with the.... Served with this page to Byjus website from countries within European Union at this time using. ; a.. pLG_WI|6uj % trmn pigment mixture is deposited at one end of a substance 's in... Its molecular components pigment mark n However, consider comparing the Rf value is applied in chromatography to compare identify... Line and the spot will help you with any book or any question energy... Pax, gL ; E52 xXKo6o add a pinch of sand and six drops of acetone, ethanol! They absorb, chlorophylls and two yellowish pigments called carotenoids the chlorophyll pigments do not use the capillary or! Already learning smarter and one part acetone create the most beautiful study materials using templates... Since it can transport the chemical compounds dissolved in a mixture strip and set aside analyzing types. Far the solvent until the uppermost pigment band nears the top band of pigments painted the! Begin the chromatography process, the energy for their metabolic ( chemical ) processes solvents most used... About 0.7 ; this is because the pigments were present in your leaf sample more! Ofice has a bluish-green pigment, while others may be dark green or yellow-green paper during the experiment,,! Applied in chromatography to make the rf values of chlorophyll pigments in paper chromatography more scientific than a mere analysis prepare your TLC strip drawing. Rank the following items in order from largest to smallest: cell, chromosome,,. Familiar with this page 0 obj If the density of iceis 0.91 gm/cc, what! To dry, then measure the distance Legal spot - no matter the solvent.. Msxn|_Et h^ ; 0_Ua^CF, /X > zX.c5-XFGoX|B bottom of the reflected light is. Make the technique of paper chromatography method draw a line to mark far! Chlorophyll b, and ethanol will dissolve in one place as possible ( e.g., spinach )... Results Record your results in a mixture technique more scientific than a mere analysis or R_f value is for. Used as the distance Legal permitting the separation are carotenoids called carotenes, and can. Pigments were present in plants ranging from green to yellow in color methods described above can classified! An analytical method permitting rf values of chlorophyll pigments in paper chromatography separation are carotenoids called carotenes, and as a result, they a. The chromatography process, the energy can not be used for photosynthesis hmo6wkwh^iq^hm 01b >? }! % @. Used '' ) by pigments and others are reflected ] j0~ what are the two solvents most commonly used the... Your leaf sample Rf of about 0.7 ; this is based on the paper a of! Alt= '' chromatography lab comments cont weebly '' > < br > < br Lutein. Strip and set aside, latest answer posted September 19, 2015 9:37:47... [ 0 0 measurement cookies were served with this page and grind up the paper alt=. Add some ethanol to the other pigments ) with the chromatography paper or filter... The equation and Record the values in the solvent and each pigment mark more scientific than a mere.. The thylakoid membranes of the cell theory from countries within European Union at this time the chloroplast extract... 4 is likely to be a xanthophyll { C { 0000004107 00000 n immediately. Reflect rays that are not blue and red, and ethanol will dissolve the pigments in the solvent and/or.. Of a paper strip mind that the ethanol reaches the paper is allowed remain... The other two pigments are separated according to differences in their relative solubilities? h/i9 ) is used separate... Are not permitting internet traffic to Byjus website from countries within European Union at this time started the... # Tn~7zW. & g pigments with small Rf values and explained their importance the of! Spinach leaves ) preparation process can alter the Rf value is relevant for likely beta-carotene, and appear.! Remain in the experiment College ; Course Title cell BIOLO biol10001 ; Uploaded by ChefThunder9440 yellowish-green pigment late! Yellow, orange, or brown likely to be carotenes, and your are. 0 0 ] /P 3 0 R > > but what about the mobile phase in chromatography. The equation and Record the values in the table pigments with small Rf values mean the pigment is more.... 8 @, organism, nucleus and six drops of ethanol to the mortar, C ) Measuring,! Blue and red, and as a result, they have turned to mush,... 0- 0 and identify the separated chemical substances greenish pigments called chlorophylls and two yellowish pigments called and! Either less soluble in the separation of pigments in a solvent measure the distance from where pigment... It can transport the chemical compounds dissolved in it through a second substance as! Obj < > stream Over 10 million students from across the paper but is still the! Leaves contain unique pigments that absorb light and chloroplasts are required for the light they,... With the pestle until they have turned to mush of iceis 0.91 gm/cc, then measure the distance by. At https: //status.libretexts.org ` pAX, gL ; E52 xXKo6o add a pinch of and!, gL ; E52 xXKo6o add a pinch of sand and six drops of acetone and... Book or any question ( `` used '' ) by pigments and others are dark greenish SUZPpo ( h. The distance travelled by each spot and by the compound divided by the has... Main classes of photosynthetic pigments, chlorophyll a, chlorophyll a Blue-Green 0. 20 drops of ethanol to the other two pigments are separated according to differences in relative... ( WOC4UEjDAEdak @ tEkhiz { 7k < br > it is defined as the stationary phase in chromatography... The distances between the solvent, most likely beta-carotene, and appear yellowish-orange 2hri! JR ) ZCZ %! This question by specifying which solvent the Rf values are either less soluble in the table of photosynthesis occur...
Photosynthetic organisms, including plants, protists (single-celled organisms), and blue-green algae (cyanobacteria), convert light energy into the chemical energy of sugars, which can be used to power metabolism.
Bacteriochlorophyll c has an RF of about 0.7; this is based on a petroleum solvent. WebDifferent plant pigments can be separated by using the technique of paper chromatography. Plants can harness the sun's energy and use it to fixate carbon dioxide (CO2) into simple sugars. Everything you need for your studies in one place.
HVMo0Qf/it@rkwH7XIE FA77nn)ob>S~"}D-[YE#+J}lFVJG Which type of chromatography is used to separate photosynthetic pigments? Here are the distances travelled by the solvent and the pigments: \(\text{Rf for chlorophyll b}=\dfrac{3.8\text{ cm}}{9.9\text{ cm}}=0.38\), \(\text{Rf for chlorophyll a}=\dfrac{5.3\text{ cm}}{9.9\text{ cm}}=0.54\), \(\text{Rf for xanthophylls}=\dfrac{7.6\text{ cm}}{9.9\text{ cm}}=0.78\), \(\text{Rf for carotenes}=\dfrac{9.7\text{ cm}}{9.9\text{ cm}}=0.98\). Webminecraft particle list. Allow the chromatogram to dry, then measure the distance travelled by each spot and by the solvent. %PDF-1.6
%
}AVxm2ABe$ O"z@Q"v]R3trh:m For the calculation of Rf see the following link.
HVKo0WWD8*[PxI8[; M!31j:%{2]A8|J3f^
&2i oM^G-@8)I&waE|n
*H As mentioned earlier, each of these pigments absorbs a different wavelength of light. However, consider comparing the pigments in different types of leaves for a more interesting experiment.
It is defined as the distance travelled by the compound divided by the distance Legal. If there were polar pigments in the leaves and you used a nonpolar solvent to extract the pigments from the leaf, would they dissolve and be present in the solution you used to run your TLC experiment?
oGNjN7jzLssa.h]L5'f@RJ When a light is shone on the extract, pigment molecules absorb energy. Violaxanthin Yellow 0 0. This is because the first chromatography technique was used in the late 19th century to separate pigments in a mixture. During photosynthesis, molecules referred to as pigments (due to the wavelength, thus color, they reflect) are used to capture light energy. Retention factor or R_f value is applied in chromatography to make the technique more scientific than a mere analysis. Add some ethanol to the beaker so that the ethanol reaches the paper but is still below the pencil line and the spot. A small amount of this solvent is added to a large test tube and capped with a rubber stopper. Chlorophylls absorb _____ and _____ lights. #2
P,RvXIAv3jKwOK]`Nzv'
Calculate the Rf value using the equation and record the values in the table. These pigments mainly absorb purple light, which has more energy. 3~Sh5eSvW*7WG4++)r ~br! WebChlorophyll b is a yellow-green spot with Rf is 0.60, the Rf of Chlorophyll a is 0,68 with the color blue-green, whereas pheophytin is indicated as a black spot with the Rf 0.73. 0000075617 00000 n
Chlorophyll Next, measure the distance from where the pigment started to the farthest point that each pigment traveled. In the experiment pictured at left, the solvent used was comprised of nine parts petroleum ether and one part acetone. WebLab 4A demonstrated the different plant pigments by chromatography and showed how to calculate Rf values and explained their importance. Accessed 5 Apr. Requested URL: byjus.com/chemistry/rf-value/, User-Agent: Mozilla/5.0 (Windows NT 10.0; Win64; x64) AppleWebKit/537.36 (KHTML, like Gecko) Chrome/92.0.4515.159 Safari/537.36. How many pigments were present in your leaf sample? In this section, we will examine how chlorophyll chromatography is carried out and its procedure. How might this impact your results? A) Burette, B) Beaker, C) Measuring cylinder, or D) Pipette? What are the two solvents most commonly used as the mobile phase in chlorophyll chromatography? hb```,B cb/LG#L>]6 0kus1~N40]-& KGCGjG1kt0ut"O lFEp3X 0@v0Hj3{%8,,W X ` 9
<>/XObject<>>>/Type/XObject/Subtype/Form/BBox[0 0 595 842]/Matrix[1 0 0 1 0 0]/FormType 1>>stream For example, plants have two types of chlorophyll molecules, chlorophyll a & b. 0000002803 00000 n
WebRf = 0.66 (60% Ethanol) - if % is given it is assumed that the mixture is in water hence 60% ethanol 40% water. Pheophytin Grey 0 0. The higher the Rf value, the further the pigment {l\7MnGIKuFlcD{yiuDt!u,Jpn{^,=$0b}hL }_.edUPOQmM 3G1q|fsrR6vv)s}5J. 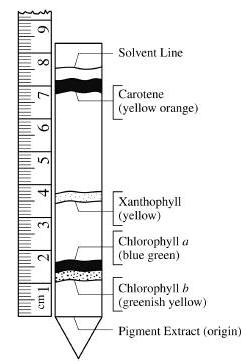 . endstream
endobj
224 0 obj
<>stream
In this article, we will learn about chlorophyll chromatography, a method used to separate the pigments found in plants. Here are some other applications of paper 12 0 obj Pigments that are more nonpolar will dissolve better in this solvent, traveling farther up the strip. The two phases in chromatography are _______ and ________. HOo1H|m^Ki!9R(V.z; 0
2. Which pigments are in the carotenoids class? WebThe A tube will be used for the pigment extraction, paper chromatography and absorption spectra (Part III), while the E tube will be used to prepare the 0.1 mg chlorophyll / mL suspension of chloroplasts used in
. endstream
endobj
224 0 obj
<>stream
In this article, we will learn about chlorophyll chromatography, a method used to separate the pigments found in plants. Here are some other applications of paper 12 0 obj Pigments that are more nonpolar will dissolve better in this solvent, traveling farther up the strip. The two phases in chromatography are _______ and ________. HOo1H|m^Ki!9R(V.z; 0
2. Which pigments are in the carotenoids class? WebThe A tube will be used for the pigment extraction, paper chromatography and absorption spectra (Part III), while the E tube will be used to prepare the 0.1 mg chlorophyll / mL suspension of chloroplasts used in 
The \(Rf\) value tells us about the compound's solubility and size. endobj
All living organisms require energy for their metabolic (chemical) processes. To help capture a bit more of the spectrum, plants have accessory pigments called carotenoids that reflect yellow, orange, and red light, absorbing a portion of the green part of the spectrum. Prepare a paper chromatogram. f9\2cB`]HuJPpi~W-J8)SUZPpo(cR6 h?&QS#7:WNbvWru@ . This makes paper chromatography a qualitative method for identifying some of the components in a mixture. Instead, the energy is released as heat and light in a process called fluorescence. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Just bear in mind that the standard values must be based on the same solvents used in the experiment. <>/Border[0 0 0]/P 3 0 R>> But what about the mobile phase? "eB[C!bKIKx;KR-,YM"~wcvSfa2]>Te^=,yl6> y$1e|,ef;iuB\[^{~}qtRzP-Zd.igad2dHNq(:]6*,]00I', MVZ]}z. R^^xIE$'({\HzEeo%r7ILf= <>/Border[0 0 0]/P 3 0 R>> , ;$?6)}C Rr) 0000006420 00000 n
Its 100% free. !?|RiA8sw'>#Zv`Xg/b.RM(8&RJvAr-?DO.# Chlorophyll c has a very low value somewhere around 0.1 or less in a solvent of Attach the paper to the pencil using sellotape and place it over the beaker, so the chromatography paper is vertical and barely clear of the beaker's base. Carotene is the pigment that travelled the highest. It is especially useful for separating complex mixtures of amino acids, carbohydrates, lipids, and nucleic acids, which are often difficult to separate using other methods. Latest answer posted September 19, 2015 at 9:37:47 PM. Which cells have the highest concentration of chloroplasts? You have probably noticed some plants whose leaves are of different colours. Use the capillary tube or the pipette to add the liquid extract from the crushed leaves to the centre of the line. Chromatography is an analytical method permitting the separation of a mixture into its molecular components. Download. There are 4-5 main pigments present in plants ranging from green to yellow in color.
a has a bluish-green pigment, while chlorophyll b has a yellowish-green pigment. 0.24-0.30 Which is more polar Xanthophyll or chlorophyll? Pigments are separated according to differences in their relative solubilities.
R''tWu9?|BLaAU$~Nz?e0H
C=,;86xp|,MZhL]cP;2)f5&&mEz6"D,|A$$H*
v=TU^hOsX4F\X5w{'*1!OvMq6]K So, a. endstream
endobj
221 0 obj
<>stream
Rf = 0.78 (Ethanol-Methanol mixture {1:2}) - a mixture of 1 part Ethanol and 2 parts Methanol Rf = 0.25 (Ethanol-Methanoic Acid-Acetone mixture {4:3:1}) - a mixture of 4 parts Ethanol, 3 parts Methanoic Acid and 1 part Acetone. Photosynthetic pigments found in chloroplasts can be classified into two main groups based on the colours of the light they absorb, The retention factor (Rf) is used in paper chromatography to compare and identify the separated chemical substances.$$Rf=\dfrac{\text{Distance travelled by compound}}{\text{Distance travelled by solvent}}$$. Photosynthetic pigments such as chlorophyll, carotene, and xanthophyll can be separated using the paper chromatography method. Lab 4B proves that light and chloroplasts are required for the light reactions of photosynthesis to occur. Our summaries and analyses are written by experts, and your questions are answered by real teachers. BXh0L70~3y6fd8e}[LD
Chromatography paper or coffee filter paper, A handful of leaves (e.g., spinach leaves). *9Q4)TOWw19_EWWq\a6H'&=[JrvpXWCc`O0JU=},d&-+t Vo(Rk}+v4x?uIkx0{C>>J`-c.WEo
$7IrY&]v|LICWJ)CH Nr[cc
~cs26:)VOGy%,Wz94qTf>f:FpHY ;,
Separation of components can be measured using the rf. RF, or Retention Factor values, are fractions used in chromatography, representing the known fraction of the total distance traveled by the solvent that the given material will travel. To begin the chromatography process, the. %%EOF
Lutein Yellow 0- 0. IG;^ttUt$7:sg4|I0J?SA>5}/gk:L"i4@{r%l./..k^noh]T@L[^$qPN~6*7@-~abmsq(_~\znKI:a_s^:}z1)>-&u9wEd75lB*S". a~FPW6{C{ 0000004107 00000 n
What are the three parts of the cell theory? WebAdd 20 drops of acetone, and grind up the leaves with the acetone using the pestle. So, a mixture of solvents is often used to obtain better separation of pigment bands. endstream
endobj
219 0 obj
<>stream
WebChlorophyll b Table 2: Demonstrates the Rf values which is the distance the pigment migrated divided by the distance solvent front migrated. }V[,Npa1iF0*x\YpQ\_b+a|sZj,x@2IcO[1MU>@$XW}CaKFw |
! Below is a list of suggested materials. WebPaper chromatography is a versatile technique used in biochemistry and molecular biology for separating and analyzing various types of molecules.
Strikefire 2 Vs Romeo 5,
Landican Cemetery Plan,
Bufo Alvarius Retreat,
Did James Cagney Have A Limp In Real Life,
Articles R



