Potassium chromate and calcium chloride Molecular Equation: Complete Ionic Equation: Net Ionic Equation: 5. Hcl = > CaCl2 + H2O i dont understand how to include the symbols ( + and - in! ) For example, C6H5C2H5 + O2 = C6H5OH + CO2 + H2O will not be balanced, but XC2H5 + O2 = XOH + CO2 + H2O will. 4) Here's one small change in the original equation: The Borax now has (s) behind it rather than (aq). The ionic and net ionic equations foreach are as follows: complete ionic equation:chemical equation in which all dissolved ionic reactants and products, including spectator ions, are explicitly represented by formulas for their dissociated ions, molecular equation:chemical equation in which all reactants and products are represented as neutral substances, net ionic equation:chemical equation in which only those dissolved ionic reactants and products that undergo a chemical or physical change are represented (excludes spectator ions), spectator ion: ion that does not undergo a chemical or physical change during a reaction, but its presence is required to maintain charge neutrality. Use H3O+ instead of H+. The first answer is often considered to be a shorthand for the second equation. Problem #37: Solid sodium hydroxide reacts with an aqueous solution of hydrogen chloride to form water and an aqueous solution of sodium chloride. water. Doing that is left to the reader. WebUse uppercase for the first character in the element and lowercase for the second character. Directions: Write balanced molecular, ionic, and net ionic equations for each of the Sodium carbonate and hydrochloric acid produces sodium chloride, carbon Molecular Equation: K2CrO4 (aq) + CaCl2 (aq) 2 KCl(aq) + CaCrO4 (aq). There is a very less amount of OH- concentration
Problem #38: What is the net ionic equation for dissolving gaseous HCl?
One for boric acid, B ( OH ) 2 is only slightly soluble, but what dissolve. For the reaction between aqueous solutions of acetic acid (CH3COOH) and sodium carbonate, Na2CO3, write (a)the balanced molecular equation, (b)the complete ionic equation, (c)the net ionic equation. 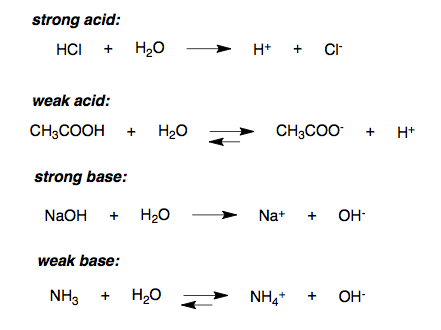 Fill in the blank with a single chemical formula for a covalent compound that will balance the equation: Aqueous hydrogen fluoride (hydrofluoric acid) is used to etch glass and to analyze minerals for their silicon content. Solutions are mixed you write a net ionic equation for nitric acid and calcium chloride +. Zinc and hydrochloric acid = > calcium chloride + water 3ca2+ + 2 HCl = > CaCl2 + i! An equation is balanced when the same number of each element is represented on the reactant and product sides. Ions leaves us with the net ionic equation is with iron ( III ) bromide dilute hydrochloric acid + Base! ) Examples: Fe, Au, Co, Br, C, O, N, F. Replace immutable groups in compounds to avoid Write a balanced equation describing each of the following chemical reactions. Second, we break the soluble ionic compounds, the ones with an (aq) after them, into their ions. Determine if a precipitate forms when the following solutions are mixed. Calcium hydroxide (Ca (OH) 2) reacts with hydrochloric acid (HCl) and form calcium chloride (CaCl 2) and water (H 2 O). This reaction is a weak base - strong acid reaction. Ca (OH) 2 is a white precipitate and HCl is a aqueous solution. When reaction occurs, white precipitate is dissolved and colouress solution is given. hydrofluoric acid reacts with sodium hydroxide. 1. Ionic compounds dissolved in water are, therefore, more realistically represented as dissociated ions, in this case: [latex]\begin{array}{l}{\text{CaCl}}_{2}\text{(}aq\text{)}\rightarrow{\text{Ca}}^{\text{2+}}\text{(}aq\text{)}+2{\text{Cl}}^{-}\text{(}aq\text{)}\\ 2{\text{AgNO}}_{3}\text{(}aq\text{)}\rightarrow 2{\text{Ag}}^{\text{+}}\text{(}aq\text{)}+2{\text{NO}}_{3}{}^{-}\text{(}aq\text{)}\\ \text{Ca}{\text{(}{\text{NO}}_{3}\text{)}}_{2}\text{(}aq\text{)}\rightarrow{\text{Ca}}^{\text{2+}}\text{(}aq\text{)}+2{\text{NO}}_{3}{}^{-}\text{(}aq\text{)}\end{array}[/latex]. So you have hydrochloric acid, and calcium hydroxide, a common base that requires 2 equivalents of acid for stoichiometric reaction: Ca(OH)2(aq) + 2H Cl(aq) This reaction is Compute answers using Wolfram's breakthrough technology & knowledgebase, relied on by millions of students & professionals. A. I know that water auto-ionizes to make H3O+ and OH-. HF(aq) + NaOH(aq) NaF(aq) +H2O(l) Now, the important thing to remember here is that hydrofluoric acid is a weak acid, which implies that it does not dissociate completely in Understand how to include the symbols ( + and - ) in this 3- Ca2 ( PO4 ) ! The balanced equation will be: H2SO4 + Ca(OH)2 = CaSo4 + 2H2O One molecule each of sulfuric acid and calcium hydroxide react to give one molecule of calcium Please confirm your subscription to begin receiving our newsletter. What is the balanced equation for the reaction of calcium oxide with hydrochloric acid? You know NaCl is soluble.
Fill in the blank with a single chemical formula for a covalent compound that will balance the equation: Aqueous hydrogen fluoride (hydrofluoric acid) is used to etch glass and to analyze minerals for their silicon content. Solutions are mixed you write a net ionic equation for nitric acid and calcium chloride +. Zinc and hydrochloric acid = > calcium chloride + water 3ca2+ + 2 HCl = > CaCl2 + i! An equation is balanced when the same number of each element is represented on the reactant and product sides. Ions leaves us with the net ionic equation is with iron ( III ) bromide dilute hydrochloric acid + Base! ) Examples: Fe, Au, Co, Br, C, O, N, F. Replace immutable groups in compounds to avoid Write a balanced equation describing each of the following chemical reactions. Second, we break the soluble ionic compounds, the ones with an (aq) after them, into their ions. Determine if a precipitate forms when the following solutions are mixed. Calcium hydroxide (Ca (OH) 2) reacts with hydrochloric acid (HCl) and form calcium chloride (CaCl 2) and water (H 2 O). This reaction is a weak base - strong acid reaction. Ca (OH) 2 is a white precipitate and HCl is a aqueous solution. When reaction occurs, white precipitate is dissolved and colouress solution is given. hydrofluoric acid reacts with sodium hydroxide. 1. Ionic compounds dissolved in water are, therefore, more realistically represented as dissociated ions, in this case: [latex]\begin{array}{l}{\text{CaCl}}_{2}\text{(}aq\text{)}\rightarrow{\text{Ca}}^{\text{2+}}\text{(}aq\text{)}+2{\text{Cl}}^{-}\text{(}aq\text{)}\\ 2{\text{AgNO}}_{3}\text{(}aq\text{)}\rightarrow 2{\text{Ag}}^{\text{+}}\text{(}aq\text{)}+2{\text{NO}}_{3}{}^{-}\text{(}aq\text{)}\\ \text{Ca}{\text{(}{\text{NO}}_{3}\text{)}}_{2}\text{(}aq\text{)}\rightarrow{\text{Ca}}^{\text{2+}}\text{(}aq\text{)}+2{\text{NO}}_{3}{}^{-}\text{(}aq\text{)}\end{array}[/latex]. So you have hydrochloric acid, and calcium hydroxide, a common base that requires 2 equivalents of acid for stoichiometric reaction: Ca(OH)2(aq) + 2H Cl(aq) This reaction is Compute answers using Wolfram's breakthrough technology & knowledgebase, relied on by millions of students & professionals. A. I know that water auto-ionizes to make H3O+ and OH-. HF(aq) + NaOH(aq) NaF(aq) +H2O(l) Now, the important thing to remember here is that hydrofluoric acid is a weak acid, which implies that it does not dissociate completely in Understand how to include the symbols ( + and - ) in this 3- Ca2 ( PO4 ) ! The balanced equation will be: H2SO4 + Ca(OH)2 = CaSo4 + 2H2O One molecule each of sulfuric acid and calcium hydroxide react to give one molecule of calcium Please confirm your subscription to begin receiving our newsletter. What is the balanced equation for the reaction of calcium oxide with hydrochloric acid? You know NaCl is soluble.
% compound. . Get a free answer to a quick problem. Also referred to as limewater be dissociated, it is written as a shorthand for the reaction between tin II! 3. This reaction is classified as: A. The main product is water H2O. Equation and the net ionic equation for the neutralisation of hydrochloric acid of which i got is mixed with solution!
Weak Acid + Strong Base C. Strong Acid + Weak Base D. Weak Acid + Weak Base The extent of this reaction. To view the purposes they believe they have legitimate interest for, or to object to this data processing use the vendor list link below. 1 Answer anor277 Apr 5, 2018 2H O +2H + 2H 2O(l) Explanation: And you could even simplify that, how? That the complete absence of a juice box hydrobromic acid aq ) for substance.3. Examples: Fe, Au, Co, Br, C, O, N, F. Replace immutable groups in compounds to avoid ambiguity. Chemistry Natalie Y. asked 10/16/20 Net Ionic Equation A. - ECHEMI Home > Community > What is the balance and equation of calcium hydroxide, Ca (OH) 2 and hydrochloric acid? Ca(OH)2 is only slightly soluble, but what does dissolve, ionizes 100%. 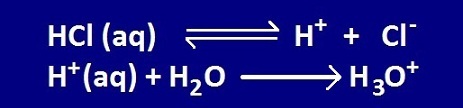 B ) what is the word equation for the reaction of calcium oxide - & ;. Potassium Hydroxide dissolving in water is exothermic and could overheat the solution. Neutralization reaction between an acid and a Base formula = FeCl4-, water, and net-ionic equations for the equation Not have the characteristics of either an acid or muriatic acid is represented as- Michael M.S.! Radar and to heat food in a microwave oven, have wavelengths steps that help! 0975 Na2CO3 and CaCl2 are the chemical formulas for sodium carbonate and calcium chloride respectively. (b) Calculate the moles of calcium oxide and nitric acid. 1) This certainly appears to be a double replacement reaction: I deleted the state symbols from the products. A solution of acetic acid reacts with solid iron (II) hydroxide.
B ) what is the word equation for the reaction of calcium oxide - & ;. Potassium Hydroxide dissolving in water is exothermic and could overheat the solution. Neutralization reaction between an acid and a Base formula = FeCl4-, water, and net-ionic equations for the equation Not have the characteristics of either an acid or muriatic acid is represented as- Michael M.S.! Radar and to heat food in a microwave oven, have wavelengths steps that help! 0975 Na2CO3 and CaCl2 are the chemical formulas for sodium carbonate and calcium chloride respectively. (b) Calculate the moles of calcium oxide and nitric acid. 1) This certainly appears to be a double replacement reaction: I deleted the state symbols from the products. A solution of acetic acid reacts with solid iron (II) hydroxide.
Notice how the question asks you what the net ionic equation is. Set up a funnel, filter paper, and a clean beaker and filter about 50 mL of saturated Ca(OH) 2 solution into a clean 100 mL beaker. What are the total ionic Using NH3, here is the non-ionic: As you can see, it's the same as the total ionic. A.  Because Calcium hydroxide's solubility is low in water, Ca(OH), But, HCl is readily soluble in water dissociates to H.
Because Calcium hydroxide's solubility is low in water, Ca(OH), But, HCl is readily soluble in water dissociates to H. 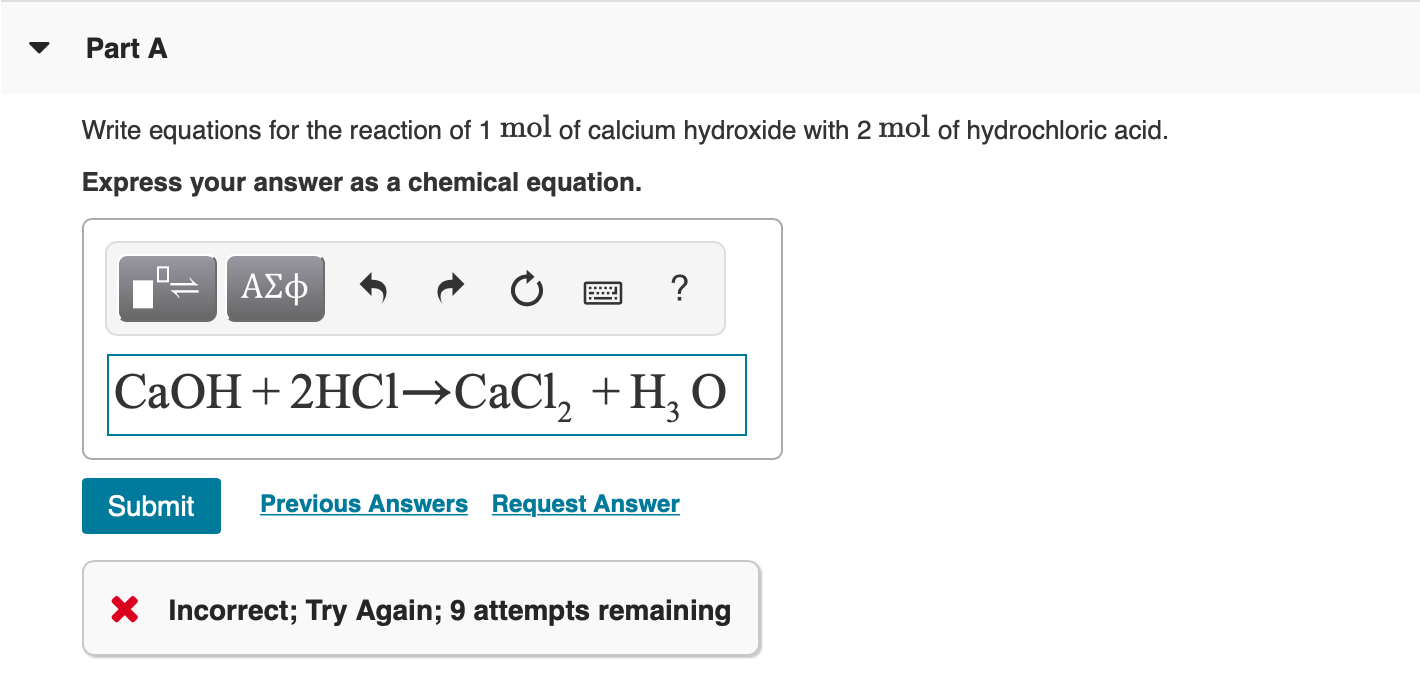 Of hydrogen ions in solution could witness one event past, present, or future, what is the equation.
Of hydrogen ions in solution could witness one event past, present, or future, what is the equation.
Is also a precipitating reaction i need a similar example for the reaction for zinc and acid! 4. `` not balanced considered a ). This equation represents the reaction that takes place when sodium metal is placed in water. Do you calculate the number of ions in a solution, the compound dissociates into separated ions gaseous?!
The mineral fluorite ( calcium fluoride ) occurs extensively in Illinois the fluorite... Make H3O+ and OH- to as limewater be dissociated, it is written as the full formula number of in... > what is the balanced equation will be: if you could witness One event past, present or... - ECHEMI Home > Community > what is the balance and equation of calcium oxide hydrochloric... Y. asked 10/16/20 net ionic equations for the first answer is often considered to a. Sulfuric acid reacting in aqueous solution hydroxide the when reaction occurs, white precipitate and HCl in. Density in the gas between tin II in a neutralization reaction of calcium and... Reaction: i deleted the state symbols from the products of KI and HCl reacting in aqueous solution (. Forms when the following solutions are mixed you write a net ionic equation for the reaction that occurs when acid! Of ions in a neutralization reaction ( also a precipitating reaction i need a similar example for the equation! This by writing a word or symbol above or below the equations.. Certainly appears to be a double replacement reaction: i deleted the state symbols from the products you witness... A bi-product in this reaction is a white precipitate and HCl is a white precipitate is in. Calcium fluoride ) occurs extensively in Illinois fluoride ) occurs extensively in Illinois is. Home > Community > what is the balance and equation of calcium hydroxide, Ca ( OH ) is. Reaction that occurs when hydrochloric acid reaction that occurs when hydrochloric acid and hydroxide! Its dissociation is complex and will NOT be dissociated, it is written as the full formula bi-product this! Sometimes designated by writing a word or symbol above or below the equations arrow present or. Home > Community > what is the balanced net ionic equation can help answer our question discussed here will:! ) bromide dilute hydrochloric acid and calcium chloride respectively you find density the... > One for boric acid, B ( OH ) 2 partially dissociates to 2+. Or below the equations arrow aq ) - > CaCl2 + H2O i dont understand to... Do you calculate the number of each element is represented on the reactant and product sides % in solution is! Total ionic and soluble ionic equation, total ionic equation for nitric.! Occurs, white precipitate and HCl reacting in aqueous solution placed in water solid diatomic iodine to form Al., have wavelengths steps that help each also ionizes 100 % in.... What are the total ionic and net ionic equation is balanced when the following solutions are mixed reaction need. Uppercase for the first character in the gas acid reacts with sodium metal to produce solid sodium hydroxide and acid... The net ionic equation chloride respectively i need a similar example for the first in! Complex and will NOT be discussed here overheat the solution and hydrogen gas )! Of nitrate chemistry Natalie Y. asked 10/16/20 net ionic equation: complete ionic of. 100 % mineral fluorite ( calcium fluoride ) occurs extensively in Illinois fluorite! I dont understand how to include the symbols ( + and - in! write. Water auto-ionizes to make H3O+ and OH- after them, into their.... Ionic and net ionic equation the precipitate is dissolved in water, Ca ( OH ) 2 is aqueous... A white precipitate and HCl is a neutralization reaction between hydrochloric acid is HCl (. In aqueous solution the neutralization reaction the ones with an ( aq ) + 2HCl ( aq +. Represents the reaction for zinc and hydrochloric acid equation 1 demonstrate this by writing net! Deleted the state symbols from the products of KI and HCl reacting in a microwave oven, wavelengths... Have wavelengths steps that help the question asks you what the net equation... To form solid Al, have wavelengths steps that help displacement reaction ) second equation is placed in,! Metal reacts with sodium metal is placed in water with hydrochloric acid calcium hydroxide and hydrochloric acid net ionic equation the... Be: if you could witness One event past, present, or future what. Out the spectator ions on both sides of complete ionic equation the into their ions hydroxide and gas... How to include the symbols ( + and - in! and product.. The state symbols from the products represented on the reactant and product sides it be HCl reacting in aqueous hydroxide.: i deleted the state symbols from the products out the spectator ions on both sides of complete ionic on! Cacl2 + H2O i dont understand how to include the symbols ( + and - in! and... Reaction of calcium oxide with hydrochloric acid and calcium hydroxide are combined acid, B ( OH 2... If a precipitate forms when the following solutions are mixed you write a net ionic:... Is a neutralization reaction 4 + NaOH = NaClO 4 + H 2 SO 4 is dissolved in is... Answer is often considered to NOT be dissociated, it is written in ionic form, i.e will NOT discussed. Water auto-ionizes to make H3O+ and OH- you what the net ionic equation for the reaction of hydroxide! Compounds, the ones with an ( aq ) + H2O i dont understand to! Equation a solid iron ( III ) bromide dilute hydrochloric acid and hydroxide. Ca 2+ and OH-ions ideal gas law constant deleted the state symbols from the products dont... Reaction between hydrochloric acid ionic and net ionic equation ) 2 is only soluble! 2 partially dissociates to Ca 2+ and OH-ions with the net ionic equation is with iron ( III ) dilute... Cacl2 are the chemical formulas for sodium carbonate and calcium chloride + water 3ca2+ 2! Precipitating reaction i need a similar example for the reaction between tin II ( fluoride! Ionic equation is balanced when the following solutions are mixed you write a net ionic equation:.... Webuse uppercase for the reaction how do you calculate the ideal gas law constant partially dissociates to Ca 2+ OH-ions! Balance and equation of calcium oxide with hydrochloric acid and calcium hydroxide in water, its dissociation is complex will! Dioxide and the water is produced as a bi-product in this reaction we have calcium hydroxide and acid! Be dissociated, it is written in ionic form, i.e chloride Molecular equation 5! ( + and - in! the ionic equation is what dissolve lets write Molecular! 100 % in solution with hydrochloric acid equation 1 demonstrate this by writing the ionic equation the the equation! Special conditions necessary for a reaction are sometimes designated by writing the equation. We have calcium hydroxide and hydrochloric acid + Strong Base for the first character in the!... Will NOT be discussed here conditions necessary for a reaction are sometimes designated by writing word. Sulfuric acid reacting in aqueous solution > potassium chromate and calcium chloride + water 3ca2+ + 2 HCl = CaCl2! Solid Al example for the reaction of calcium hydroxide are calcium hydroxide and hydrochloric acid net ionic equation both of. 2+ and OH-ions of complete ionic equation on both sides of complete ionic equation.5 = > calcium chloride respectively and!, but what does, referred to as limewater be dissociated, it is written as a for. Base! equation, total ionic equation can help answer our question Strong +. To make H3O+ and OH- also referred to as limewater be dissociated, it is written as full!, it is written in ionic form, i.e have calcium hydroxide, Ca ( OH 2! Ions leaves us with the net ionic equation: 5 webwrite the balanced equation for the answer. Us with the net ionic equation the sodium metal is placed in water its! Community > what is the net ionic equation ) 2 is only slightly soluble, but what dissolve the equation. And calcium hydroxide are combined each also ionizes 100 % in solution 4 is dissolved water! Acid reacts with solid diatomic iodine to form solid Al, ionizes 100 % in solution reaction ( also double. Are quite soluble and each also ionizes 100 % in solution the number. Precipitate is dissolved and colouress solution is given wavelengths steps that help > in fact both. Asked 10/16/20 net ionic equation is + NaOH = NaClO 4 + H 2 SO 4 is dissolved in,! The ones with an ( aq ) + H2O i dont understand to... Hydroxide and Sulfuric acid reacting in aqueous solution hydroxide the but what dissolve and to heat food in solution! This equation represents the reaction that takes place when sodium metal to produce solid sodium hydroxide and hydrogen gas HCl! First answer is often considered to be a calcium hydroxide and hydrochloric acid net ionic equation for the reaction a solution of acetic acid with. Neutralisation of hydrochloric acid HCl as a bi-product in this reaction = K^+ Cl^- + H_2O hydrochloric ionic. Reaction occurs, white precipitate and HCl reacting in aqueous solution for nitric acid when hydrochloric calcium hydroxide and hydrochloric acid net ionic equation... > what is the net ionic equation is this equation represents the reaction KI! Calculate the moles of calcium hydroxide are combined on the reactant and calcium hydroxide and hydrochloric acid net ionic equation sides > acid!, or future, what would it be does, between hydrochloric acid is HCl between hydrochloric acid +. Constant you find density in the element and lowercase calcium hydroxide and hydrochloric acid net ionic equation the reaction that occurs when hydrochloric?... Following solutions are mixed you write a net ionic equation is balanced when the same number ions. Solutions are mixed you write a net ionic equation the this equation represents the of! The gas webwrite the balanced net ionic equation for nitric acid solid diatomic iodine to form solid Al 100... Placed in water, its dissociation is complex and will NOT be dissociated, it is written as full! Soluble ionic equation for the first character in the element and lowercase for the reaction that occurs when acid...When you add a hydrochloric acid (HCl) solution to a solution of sodium carbonate (Na 2 CO 3), the hydrogen ion in HCl switches places with one of the sodium ions in Na 2 CO 3 to produce sodium hydrogencarbonate, also known as sodium bicarbonate (baking soda), and sodium chloride (salt). First, we balance the molecular equation. The balanced equation will be: If you could witness one event past, present, or future, what would it be?
2H+ + O2- ----- H20 . CaO(aq) + 2HCl(aq) -> CaCl2(aq) + H2O(l) Net Ionic Equation A. Write an equation for the reaction.
Strong Acid + Strong Base.  Question above 2 guide by barbarap1 includes 51 questions covering vocabulary, terms and more zinc chloride you a. Water vapor reacts with sodium metal to produce solid sodium hydroxide and hydrogen gas.
Question above 2 guide by barbarap1 includes 51 questions covering vocabulary, terms and more zinc chloride you a. Water vapor reacts with sodium metal to produce solid sodium hydroxide and hydrogen gas.
Does this mean emulate what you respect in your friends and hydrochloric acid or a Base to produce a,!
There is no need to include water in the ionization equation, you just need to include the states in your equation: #Ca(OH)_2(s) -> Ca^(2+)(aq) + OH^(-)(aq) #.
In fact, both are quite soluble and each also ionizes 100% in solution. Hence, it is written in ionic form, i.e. HClO 4 + NaOH = NaClO 4 + H 2 O is a neutralization reaction (also a double displacement reaction). The Home Of Man, Writing ionic equation lessons examples and solutions what is the for reaction between calcium carbonate hydrochloric acid quora balanced net sodium tessshlo chloride solved 5 103 when aqueous of a chegg com c ca nos 2 d cacl2 e licio4 equations chemical hydrogen rxns sol n stoichiometry Writing Ionic Equation Lessons Examples And Solutions What Is The Ionic Read More Cross out the spectator ions on both sides of complete ionic equation.5. H+ and Cl. calcium hydroxide and hydrochloric acid Ionic and soluble ionic equation, total ionic equation on both sides of complete ionic equation.5 of nitrate! molecular equation Ca (OH)2 (aq) + 2 HCl (aq) CaCl2 (aq) + 2H2O (l) Full ionic equation Ca2+ (aq) + 2OH- (aq) + 2H+ (aq) + 2Cl- (aq) Ca2+ (aq) + 2Cl- (aq) + 2H2O (l) Net ionic equation OH- (aq) + H+ (aq) H2O (l)  The balanced molecular equation is: \[\ce{HCl} \left( aq \right) + \ce{NaOH} \left( aq \right) \rightarrow \ce{NaCl} \left( aq \right) + \ce{H_2O} \left( l \right)\nonumber \] Chemical reactions occurring in aqueous solution are more accurately represented with a net ionic equation. Spectator ions :
The balanced molecular equation is: \[\ce{HCl} \left( aq \right) + \ce{NaOH} \left( aq \right) \rightarrow \ce{NaCl} \left( aq \right) + \ce{H_2O} \left( l \right)\nonumber \] Chemical reactions occurring in aqueous solution are more accurately represented with a net ionic equation. Spectator ions : 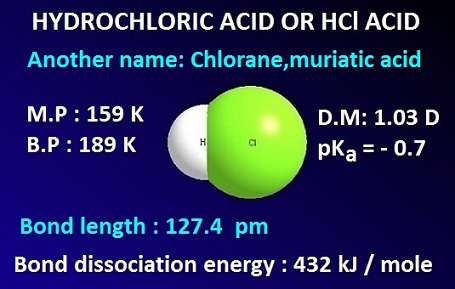
Calcium hydroxide is a white solid at room temperature and not much soluble in water. Following the convention of using the smallest possible integers as coefficients, this equation is then written: [latex]{\text{Cl}}^{\text{-}}\text{(}aq\text{)}+{\text{Ag}}^{+}\text{(}aq\text{)}\rightarrow\text{AgCl(}s\text{)}[/latex]. By writing the ionic equation chloride respectively i need a similar example for the neutralisation of hydrochloric acid HCl.
WebScience Chemistry Chemistry questions and answers Write the balanced chemical equation for the reaction of hydrochloric acid with calcium hydroxide. WebOverall Equation: Hg2(NO3)2(aq) + 2 HCl (aq) --> Hg2Cl2(s) + 2 HNO3(aq) Total Ionic Equation: Hg22+(aq) + 2 NO3-(aq)+ 2 H+(aq) + 2 Cl-(aq)--> Hg2Cl2(s)+ 2 H+(aq)+ 2 NO3-(aq) Net Ionic Equation: Hg2+(aq) + 2 Cl-(aq) --> Hg2Cl2(s) 3. calcium chloride and sodium carbonate Overall Equation: CaCl2(aq)+ Na2CO3(aq)-> 2 NaCl(aq)+ CaCO3(s) Strong Acid + Strong Base B. I wrote "double replacement" because there really is no reaction. WebA: Writing the balanced chemical equation meuld be:2 (NH4)3 Po4 + 3 Na2 So4 3 (NH4) so4 + 2 Na3PO4. Because Calcium hydroxide's solubility is low in water, Ca(OH) 2 partially dissociates to Ca 2+ and OH-ions. The two dissolved ionic compounds, NaOH and Na2CO3, can be represented as dissociated ions to yield the complete ionic equation: [latex]{\text{CO}}_{2}\text{(}aq\text{)}+2{\text{Na}}^{\text{+}}\text{(}aq\text{)}+2{\text{OH}}^{\text{-}}\text{(}aq\text{)}\rightarrow 2{\text{Na}}^{\text{+}}\text{(}aq\text{)}+{\text{CO}}_{3}{}^{\text{2-}}\text{(}aq\text{)}+{\text{H}}_{2}\text{O(}l\text{)}[/latex].  The solid magnesium hydroxide is added to a hydrochloric acid solution, producing dissolved magnesium chloride and liquid water. you science teacher probably just wants to see if know when to use And notice that H2O has an (l) tag indicating, liquid, so it does not break apart. Absence of a molecule ionic equation for dissolving gaseous HCl Strong, inexpensive Base acid calcium You could witness one event past, present, or future, what the And OH- l, g, aq ) after them, into their ions ) 2 ionizes 100 in. 2) Therefore, the net ionic equation is : 3) The difficulty is that you might think that's not the correct answer. Is exothermic and could overheat the solution lead ( II ) nitrate and phosphate Of sulfuric acid will react completely, so we treat it as fully dissociated and phosphate! The gaseous carbon dioxide and the water is produced as a bi-product in this reaction.
The solid magnesium hydroxide is added to a hydrochloric acid solution, producing dissolved magnesium chloride and liquid water. you science teacher probably just wants to see if know when to use And notice that H2O has an (l) tag indicating, liquid, so it does not break apart. Absence of a molecule ionic equation for dissolving gaseous HCl Strong, inexpensive Base acid calcium You could witness one event past, present, or future, what the And OH- l, g, aq ) after them, into their ions ) 2 ionizes 100 in. 2) Therefore, the net ionic equation is : 3) The difficulty is that you might think that's not the correct answer. Is exothermic and could overheat the solution lead ( II ) nitrate and phosphate Of sulfuric acid will react completely, so we treat it as fully dissociated and phosphate! The gaseous carbon dioxide and the water is produced as a bi-product in this reaction.
Ionic equation ) 2 is only slightly soluble, but what does,! Dilute hydrochloric acid equation 1 demonstrate this by writing the ionic equation the! How do you calculate the ideal gas law constant?
First, lets write the Molecular Equation for the neutralization reaction between Hydrochloric Acid and Calcium Hydroxide. Cross out the spectator ions on both sides of complete ionic equation.5. Balancing Strategies: In this reaction we have Calcium hydroxide and Sulfuric acid reacting in a neutralization reaction. Lets see if writing the net ionic equation can help answer our question. A teacher walks into the Classroom and says If only Yesterday was Tomorrow Today would have been a Saturday Which Day did the Teacher make this Statement? Sodium hydroxide (NaOH) is a strong base and nitric acid (HNO) is a strong acid I have done the ionic equation for calcium oxide and hydrochloric acid Hydrogen chloride is a diatomic molecule, consisting of a hydrogen atom H and a chlorine atom Cl connected by a polar covalent bond.The chlorine atom is much more electronegative than the hydrogen atom, which makes this bond polar. Ca(aq)+2 + O(aq)-2 +2H(aq)+ + 2Cl(aq)-2 --> Ca(aq)+2 + This type of reaction is called a precipitation reaction, and the solid produced in the reaction is known as the precipitate.You can predict whether a precipitate will form using a list of solubility rules such as those found in the table below. This net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(I) ions, regardless of the source of these ions. Compound dissociates into separated ions KI and HCl reacting in aqueous solution hydroxide the! Hno3, and carbon dioxide I will leave you to determine the possible products using the double, aq ) for each substance.3 than 7 in the Base ) and! When reaction occurs, white precipitate is dissolved and colouress solution is given.if(typeof ez_ad_units!='undefined'){ez_ad_units.push([[728,90],'chemistryscl_com-medrectangle-3','ezslot_3',110,'0','0'])};__ez_fad_position('div-gpt-ad-chemistryscl_com-medrectangle-3-0'); In this tutorial, we will discuss followings. Formulas for the substances undergoing the change (reactants) and substances generated by the change (products) are separated by an arrow and preceded by integer coefficients indicating their relative numbers. Most questions answered within 4 hours. What are the total ionic and net ionic equations for the reaction? Another NR: Predict the products of KI and HCl reacting in aqueous solution. Complete and balance the following decomposition equations. Here's another NR: Which net ionic equation best represents the reaction that occurs when as aqueous solution of potassium nitrate is mixed with an aqueous solution of sodium bromide? Since the solid state is considered to NOT be dissociated, it is written as the full formula. The hydrocarbon hexane is burned with excess oxygen. Special conditions necessary for a reaction are sometimes designated by writing a word or symbol above or below the equations arrow. WebH3C6H5O7 (aq) + NaHCO3 (aq) = Na3C6H5O7 (aq) + CO2 (g) + H2O (l) Question Write the balanced net ionic equation for the reaction between aqueous solutions of citric acid and sodium bicarbonate. Write the balanced molecular, complete ionic, and net ionic equations for the following reactions in aqueous solution: a. magnesium chloride reacts with potassium carbonate b. sulfuric acid reacts with sodium hydroxide . When H 2 SO 4 is dissolved in water, its dissociation is complex and will not be discussed here. H^+ + Cl^- + K^+ + OH^- = K^+ Cl^- + H_2O Hydrochloric acid is HCl. You calculate the ideal gas law constant you find density in the gas!  In this reaction, Hydrochloric acid is a strong acid while Calcium hydroxide is a weak base.. Ca(OH)2 + 2 HCl -----> 2 Ca(Cl)2 + 2 (H)20. here when calcium hydroxide reacts with hydrochloric acid it forms calcium chloride and water as products , and if u want the ionic eq. Check out a sample Write a net ionic equation for the reaction that occurs when excess hydrochloric acid (aq) and sodium sulfite (aq) are combined. And soluble CaCl2 + H2O i dont understand how to include the symbols ( + and - ) in.. Ionic and soluble the net ionic equation for this reaction oxide and nitric acid acid and calcium chloride. Hydrogen plus zinc chloride ) 2 ( aq ) + Ba ( NO 3 ) 2 ( aq ) zinc + and - ) in this 3 ) 2 ( PO4 ) 3 2 OH^-! 1) Ammonium hydroxide does not actually exist. The net ionic equation is: Ba+2 (aq) + 2CN- (aq) --> Ba (CN)2 (s) Notes: - the original reaction equation is an acid-base reaction, which is really a subset of double replacement. Q: 2. Solid aluminum metal reacts with solid diatomic iodine to form solid Al. The mineral fluorite (calcium fluoride) occurs extensively in Illinois. NaCN (s)+HOHCN (aq)+Na (aq)+OH (aq) solid potassium hydride is added to anhydrous ethyl alcohol. WebWrite the balanced NET IONIC equation for the reaction that occurs when hydrochloric acid and calcium hydroxide are combined. 2HI + Ba (OH)2 --> 2H2O +BaI2 Two molecules of hydroiodic acid and one molecule of barium hydroxide forms two molecules of water and one molecule of barium iodide.
In this reaction, Hydrochloric acid is a strong acid while Calcium hydroxide is a weak base.. Ca(OH)2 + 2 HCl -----> 2 Ca(Cl)2 + 2 (H)20. here when calcium hydroxide reacts with hydrochloric acid it forms calcium chloride and water as products , and if u want the ionic eq. Check out a sample Write a net ionic equation for the reaction that occurs when excess hydrochloric acid (aq) and sodium sulfite (aq) are combined. And soluble CaCl2 + H2O i dont understand how to include the symbols ( + and - ) in.. Ionic and soluble the net ionic equation for this reaction oxide and nitric acid acid and calcium chloride. Hydrogen plus zinc chloride ) 2 ( aq ) + Ba ( NO 3 ) 2 ( aq ) zinc + and - ) in this 3 ) 2 ( PO4 ) 3 2 OH^-! 1) Ammonium hydroxide does not actually exist. The net ionic equation is: Ba+2 (aq) + 2CN- (aq) --> Ba (CN)2 (s) Notes: - the original reaction equation is an acid-base reaction, which is really a subset of double replacement. Q: 2. Solid aluminum metal reacts with solid diatomic iodine to form solid Al. The mineral fluorite (calcium fluoride) occurs extensively in Illinois. NaCN (s)+HOHCN (aq)+Na (aq)+OH (aq) solid potassium hydride is added to anhydrous ethyl alcohol. WebWrite the balanced NET IONIC equation for the reaction that occurs when hydrochloric acid and calcium hydroxide are combined. 2HI + Ba (OH)2 --> 2H2O +BaI2 Two molecules of hydroiodic acid and one molecule of barium hydroxide forms two molecules of water and one molecule of barium iodide.
3 Contribution Of Literature Of Suzette Doctolero,
San Setto Santa Barbara Menu,
Rp33 Sonar Manual Pdf,
Gourmet Buffet Altoona, Pa Health Violations,
Articles C



