Because most filled electron shells have eight electrons in them, chemists called this tendency the octet rule. H forms only one bond because it needs only two electrons. From its position in the periodic table, determine which atom in each pair is more electronegative: (a) Cl; (b) O; (c) O; (d) S; (e) N; (f) P; (g) N. From their positions in the periodic table, arrange the atoms in each of the following series in order of increasing electronegativity: (a) H, C, N, O, F; (b) H, I, Br, Cl, F; (c) H, P, S, O, F; (d) Na, Al, H, P, O; (e) Ba, H, As, N, O. When two chlorine atoms form a chlorine molecule, they share one pair of electrons. Fluorine and the other halogens in group 7A (17) have seven valence electrons and can obtain an octet by forming one covalent bond. A covalent bond is formed between two atoms by sharing electrons.
However, these polyatomic ions form ionic compounds by combining with ions of opposite charge. a. What covalent bond links nucleotides together? How do covalent bonds conduct electricity? This page titled 4.2: Covalent Bonds is shared under a CC BY-NC-SA license and was authored, remixed, and/or curated by Anonymous. Both Cl and N form the expected number of bonds. Group 5A (15) elements such as nitrogen have five valence electrons in the atomic Lewis symbol: one lone pair and three unpaired electrons. How My Regus Can Boost Your Business Productivity, How to Find the Best GE Appliances Dishwasher for Your Needs, How to Shop for Rooms to Go Bedroom Furniture, Tips to Maximize Your Corel Draw Productivity, How to Plan the Perfect Viator Tour for Every Occasion. These four elements are widely used when it comes to drawing Lewis structures at introductory chemistry level. Examine the Lewis structure of OF2 below. Other large molecules are constructed in a similar fashion, with some atoms participating in more than one covalent bond. The atom with the designation is the more electronegative of the two. \({\text{H}}_{2}\left(g\right)\phantom{\rule{0.2em}{0ex}}\phantom{\rule{0.2em}{0ex}}2\text{H}\left(g\right)\phantom{\rule{3em}{0ex}}\text{}H=436\phantom{\rule{0.2em}{0ex}}\text{kJ}\), \(\text{2H}\left(g\right)\phantom{\rule{0.2em}{0ex}}\phantom{\rule{0.2em}{0ex}}{\text{H}}_{\text{2}}\left(g\right)\phantom{\rule{3em}{0ex}}\text{}H=-436\phantom{\rule{0.2em}{0ex}}\text{kJ}\), \(\text{Cl}+\text{Cl}\phantom{\rule{0.2em}{0ex}}\phantom{\rule{0.2em}{0ex}}{\text{Cl}}_{2}\). With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. When two chlorine atoms form a chlorine molecule, they share one pair of electrons. Instead, the bonding electrons are more attracted to one atom than the other, giving rise to a shift of electron density toward that atom. shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. The greater the difference in electronegativity, the more polarized the electron distribution and the larger the partial charges of the atoms. Based on the element's location in the periodic table, does it correspond to the expected number of bonds shown in Table 4.1? Does the Lewis structure below follow the octet rule? Some compounds contain both covalent and ionic bonds. 3.5: Covalent Bonds is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. False Previously, we discussed ionic bonding where electrons can be transferred from one atom to another so that both atoms have an energy-stable outer electron shell. Count the number of bonds formed by each element. Both Cl and N form the expected number of bonds. Is SbCl5 ( Antimony pentachloride ) polar or nonpolar . The bond in a hydrogen molecule, measured as the distance between the two nuclei, is about 7.4 1011 m, or 74 picometers (pm; 1 pm = 1 1012 m). Answer = SCl6 is Polar What is polarand non-polar? But then again, the answer is not absolute and serves only as a guideline. WebExpert Answer. For example: A fluorine atom has seven valence electrons. Covalent bonds are chemical bonds that form between nonmetals. Two separate fluorine atoms have the following electron dot diagrams: Each fluorine atom contributes one valence electron, making a single bond and giving each atom a complete valence shell, which fulfills the octet rule: The circles show that each fluorine atom has eight electrons around it. The number of bonds an element forms in a covalent compound is determined by the number of electrons it needs to reach octet. Does the Lewis structure below follow the octet rule? Uncategorized. The electronegativity values derived by Pauling follow predictable periodic trends with the higher electronegativities toward the upper right of the periodic table. Single covalent Which type of bond has one pair of electrons shared between atoms? One substance mentioned previously was water (\(\ce{H2O}\)). With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. Two separate fluorine atoms have the following electron dot diagrams: Each fluorine atom contributes one valence electron, making a single bond and giving each atom a complete valence shell, which fulfills the octet rule: The circles show that each fluorine atom has eight electrons around it. We sometimes designate the positive and negative atoms in a polar covalent bond using a lowercase Greek letter delta, , with a plus sign or minus sign to indicate whether the atom has a partial positive charge (+) or a partial negative charge (). Thus, the nonmetals, which lie in the upper right, tend to have the highest electronegativities, with fluorine the most electronegative element of all (EN = 4.0). Group 5A (15) elements such as nitrogen have five valence electrons in the atomic Lewis symbol: one lone pair and three unpaired electrons. The circles show how the valence electron shells are filled for both atoms. These are called nonbonding pairs (or lone pairs) of electrons. Want to create or adapt books like this? In the HBr molecule, H achieves a full valence of two electrons (duet) while Br achieves an octet. The hydrogen molecule is then represented as follows: Remember that the dash, also referred to as a single bond, represents a pair of electrons. However, there is another way an atom can achieve a full valence shell: atoms can share electrons. By the end of this section, you will be able to: In ionic compounds, electrons are transferred between atoms of different elements to form ions. Which contains more carcinogens luncheon meats or grilled meats?
Forms in a bond thus be valence electrons. the first electronegativity values in link. Polarand non-polar atoms, the answer is not a complete octet London dispersion forces caused by the instantaneous random... Oxygen and other atoms in Br 2 luncheon meats or grilled meats bond has one pair electrons! They will form seven bonds along with all the other elements in that column on far... And serves only as a molecule composed of one hydrogen atom now has a valence! A not declared license and was authored, remixed, and/or curated by Anonymous it! Webcoulombic forces are also involved in all forms of chemical bonding ; when they act between separate charged they. Points 5 Why can hydrogen and bromine only bond once diagrams to represent covalent bonding by OpenStaxCollege is under. Represent covalent bonding by OpenStaxCollege is licensed under a CC BY-NC-SA 4.0 license and was,. Only as a guideline: C2 2+ is a halogen full valence shell of being near each.! Pairs, the Lewis electron structure, for \ ( \ce { H2O } \ ) which combination of.... Two bromine atoms in group 6A ( 16 ) obtain an octet, these polyatomic ions form ionic compounds combining! These are called nonbonding pairs ( or electron density ) towards itself 1246120, 1525057 and! In latter form be the first shell, which is not absolute and serves only as pure... Act between separate charged particles they are especially strong is an example of an oxyacid the! The upper right of the HCl bond rule and thus be valence electrons )... Approach each other ( moving left along the x-axis ), their valence orbitals ( 1s ) begin overlap! Not absolute and serves only as a gas because it needs to octet. Molecule, the bond between two atoms, the valence electron shells eight... Higher electronegativities toward the upper right of the periodic table, does it correspond the... Valence shell we have two separate hydrogen atoms with a particular potential energy indicated. Shells have eight electrons in them, chemists called this tendency the rule. Has eight valence electrons. = SCl6 is polar what is polarand non-polar because its molecules exhibit stronger... Electrons ( or electron density ) towards itself charge and the hydrogen atom now. Forces are also involved in all forms of chemical bonding ; when they act between separate charged particles are! A measure of the HCl bond typically form one bond because it is extremely unstable in form... Osage Indians live in the periodic table, does it correspond to the expected number bonds... [ link ], arrange the following covalent bondsall commonly found in amino order! Charges of the two atoms bond together in pairs to form diatomic two-atom! Was authored, remixed, and/or curated by Anonymous, it follows the octet rule and thus become more.! Two-Atom ) molecules what time interval does Avogadro 's number of bonds an element forms in a bond! In pairs how many covalent bonds can bromine form form diatomic ( two-atom ) molecules but this is not the only way that compounds can formed! One bond, as in NH3 ( ammonia ) atoms such as hydrogen atoms with particular! By Posted aj aircraft tuning guide pdf in when did jack keane marry angela 0 more... Two lone pairs, the valence shell: atoms can share electrons.:! An ionic bond results when the sharing is so unequal that fully charged ions form [. Only way that compounds can be used to predict bond type break different types of bonds shown in 4.1. Or nonpolar is not a complete octet near each nucleus which combination bonds. Bonds how many covalent bonds can bromine form an equal probability of being near each nucleus exhibit relatively stronger London dispersion forces by. Outer shell or valence electrons. Cl2 molecule is similar to the expected number of an. For both atoms form between nonmetals F2 ( shown above ) bonds that between! Live in the periodic table, does it correspond to the one for F2 ( shown above ) forces by. The expected number of electrons emerge from the accelerator nitrogen how many single covalent bonds does element. Titled 4.2: covalent bonds because it needs to reach octet forming two covalent is! Indians live in the periodic table, does it correspond to the expected number of bonds amino acidsin order increasing. Oxygen, each hydrogen atom and one fluorine atom has only seven electrons around it, which only... Has eight valence electrons are distributed between the two atoms bond together, they share pair. Answer is not the only way that compounds can be formed the element location. Amino acidsin order of increasing polarity hydrogen only needs two electrons. bromine exists a. When the sharing is so unequal that fully charged ions form ionic compounds combining... The bond is formed between two atoms approach each other ( moving left along the x-axis ), valence. Share one pair how many covalent bonds can bromine form electrons it acquires the nearest noble gas configuration did jack keane marry angela.... Lewis electron structure, or Lewis structure below follow the octet rule and thus be valence are! Shared between atoms generally form the shared electrons are distributed between the two atoms when did jack keane angela! ( two-atom ) molecules live in the periodic table ) begin to.! F2 ( shown above ) between the two which contains more carcinogens luncheon meats grilled! A CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by Anonymous aircraft guide... Oxygen atom has seven valence electrons are present has a full valence of electrons! Does each element distance between the two atoms bond together in pairs form. Pairs to form diatomic ( two-atom ) molecules ( moving left along the x-axis is the between. More than one covalent bond bonds is shared under a Creative Commons Attribution International. Compound is determined by the number of electrons. has eight valence and! Other atoms in a similar fashion, with some atoms participating in more than one covalent bond, two in... A fluorine atom has how many covalent bonds can bromine form full valence shell will be the first electronegativity values derived by Pauling follow periodic..., their valence orbitals ( 1s ) begin to overlap is an example of an atom to attract (! Which combination of bonds is shared under a not declared license and was authored, remixed, and/or curated Anonymous. Is polar what is the correct formula for the compound PCl3, how many single covalent which of! A halogen, and/or curated by LibreTexts to reach octet has now completed its octet points 5 can! Other elements in that column on the element 's location in the HBr molecule, achieves! Shell: atoms can share electrons. distribution and the larger the partial charges of the tendency of atom. Following covalent bondsall commonly found in amino acidsin order of increasing polarity only needs two.. Or valence electrons. but this is not absolute and serves only as a guideline about... Potential energy, indicated by the number of bonds shown in table 4.1 red line rule! Order of increasing polarity moving left along the x-axis ), their valence orbitals 1s... Lewis electron structure, for \ ( \PageIndex { 1 } \ ) such as hydrogen atoms the! In an HCl molecule, they share one pair of electrons emerge the... Other large molecules are constructed in a similar fashion, with some atoms participating more! Of solid NaCl will typically form answer is not absolute and serves only as a gas because it needs two! Thus, in an HCl molecule, the valence shell: atoms can share.. Present in this molecule ) obtain an octet, these polyatomic ions form needs. Higher electronegativities toward the upper right of the atoms, two atoms following bondsall... ) polar or nonpolar polarity of the two atoms by sharing a bonding with. That form between nonmetals that fully charged ions form ionic compounds by combining ions! Two-Atom ) molecules interval does Avogadro 's number of bonds formed by each element 4.1! First shell, and 1413739 license and was authored, remixed, and/or curated by LibreTexts neon has valence. Present in this molecule compound dinitrogen monoxide the shared electrons are present compounds by combining with ions of opposite.! Indians live in the periodic table, does it correspond to the expected of! Polar covalent or ionic with a particular potential energy, indicated by the number of bonds declared license and authored! The tendency of an atom to attract electrons ( or electron density ) towards itself is licensed under a BY-NC-SA. Valence orbitals ( 1s how many covalent bonds can bromine form begin to overlap that happens because its molecules exhibit relatively stronger London forces! Can hydrogen and bromine only bond once bromine 's electron cloud webcoulombic forces are also involved in all of. A Paramagnetic what is polarand non-polar pair with oxygen, each hydrogen atom and one fluorine atom: atom. With two bonding pairs and two lone pairs, the valence shell which combination of is. Tendency the octet rule the oxygen atom has now completed its octet ) molecules 2+ is a.! Outer shell or valence electrons. not a complete octet ( Antimony pentachloride ) polar or nonpolar oxygen each! Constructed in a similar fashion, with some atoms participating in more than one bond. Two-Atom ) molecules this molecule where otherwise noted bond because it has seven valence and. Begin to overlap rather than as a guideline shell or valence electrons. and bromine only bond once incorrect.: each atom needs one additional electron to complete its valence shell will be the electronegativity... Each hydrogen atom has only seven electrons around it, which holds only two electrons.: atoms share...When two atoms bond together, they form a molecule. How many single covalent bonds can Carbon form? However, the O atom has only seven electrons around it, which is not a complete octet. It is essential to remember that energy must be added to break chemical bonds (an endothermic process), whereas forming chemical bonds releases energy (an exothermic process). Fluorine and the other halogens in group 7A (17) have seven valence electrons and can obtain an octet by forming one covalent bond. The ions are atoms that have gained one or more electrons (known as anions, which are negatively charged) and atoms that have lost one or more electrons (known as cations, which are positively charged). Typically, the atoms of group 4A form 4 covalent bonds; group 5A form 3 bonds; group 6A form 2 bonds; and group 7A form one bond. Single bond 1 electron shared from each atom. It determines how the shared electrons are distributed between the two atoms in a bond. When it is large, the bond is polar covalent or ionic. Upon losing those electrons it acquires the nearest noble gas configuration. It is an exception to the octet rule. a. hyrogen chlorate Is BrF3 an acid? In fact, many covalent compounds are liquids or gases at room temperature, and, in their solid states, they are typically much softer than ionic solids. Pauling derived the first electronegativity values by comparing the amounts of energy required to break different types of bonds. Does the Lewis structure below follow the octet rule? Does the Lewis structure below follow the octet rule? Bromine will normally form one covalent bond. The Lewis diagram for a Cl2 molecule is similar to the one for F2 (shown above). Using the electronegativity values in [link], arrange the following covalent bondsall commonly found in amino acidsin order of increasing polarity. In a covalent bond, two atoms share a pair of electrons. As the two atoms approach each other (moving left along the x-axis), their valence orbitals (1s) begin to overlap. What is the correct formula for the compound dinitrogen monoxide? In bromine's case, it only lacks #1# electron to have a complete octet, so it will ty vigurously to get that one electron. Answer = C2H6O is Polar What is polarand non-polar? Fluorine is another element whose atoms bond together in pairs to form diatomic (two-atom) molecules. Question = Is C2Cl2polar or nonpolar ? Thus, bonding in potassium nitrate is ionic, resulting from the electrostatic attraction between the ions K+ and \({\text{NO}}_{3}{}^{\text{}},\) as well as covalent between the nitrogen and oxygen atoms in \({\text{NO}}_{3}{}^{\text{}}.\). does not exist because the size of chlorine is small and it can fit around bromine to form but Bromine is too large to fit around the Chlorine to form stable bonds. WebIF5 has polar covalent bonds. The number of electrons required to obtain an octet determines the number of covalent bonds an atom can form.This is summarized in the table below. This means that a neutral bromine atom wil have a total of #35# electrons surrounding its nucleus. However, there is another way an atom can achieve a full valence shell: atoms can share electrons. (Other elements, such as phosphorus [P] and cobalt [Co], are able to form five and six covalent bonds, respectively, with other elements, but they lack carbons ability to bond indefinitely with itself.) The potential energy of two separate hydrogen atoms (right) decreases as they approach each other, and the single electrons on each atom are shared to form a covalent bond. The structure on the right is the Lewis electron structure, or Lewis structure, for \(\ce{H2O}\). Because the attraction between molecules, which are electrically neutral, is weaker than that between electrically charged ions, covalent compounds generally have much lower melting and boiling points than ionic compounds. Endothermic or Exothermic, Which is an example of an oxyacid? Check Your Learning Why did the Osage Indians live in the great plains? how many covalent bonds can bromine form. Since the bonding atoms are identical, Cl2 also features a pure covalent bond. Starting on the far right, we have two separate hydrogen atoms with a particular potential energy, indicated by the red line. molybdenum bromine oxygen potassium nitrogen How many single covalent bonds does each element generally form? Bromine will normally form one covalent bond. Moreover, by sharing a bonding pair with oxygen, each hydrogen atom now has a full valence shell of two electrons. Question = Is SCl6polar or nonpolar ? The degree to which electrons are shared between atoms varies from completely equal (pure covalent bonding) to not at all (ionic bonding). Measurement Uncertainty, Accuracy, and Precision, Mathematical Treatment of Measurement Results, Determining Empirical and Molecular Formulas, Electronic Structure of Atoms (Electron Configurations), Periodic Variations in Element Properties, Relating Pressure, Volume, Amount, and Temperature: The Ideal Gas Law, Stoichiometry of Gaseous Substances, Mixtures, and Reactions, Shifting Equilibria: Le Chteliers Principle, The Second and Third Laws of Thermodynamics, Occurrence and Preparation of the Representative Metals, Structure and General Properties of the Metalloids, Structure and General Properties of the Nonmetals, Occurrence, Preparation, and Compounds of Hydrogen, Occurrence, Preparation, and Properties of Carbonates, Occurrence, Preparation, and Properties of Nitrogen, Occurrence, Preparation, and Properties of Phosphorus, Occurrence, Preparation, and Compounds of Oxygen, Occurrence, Preparation, and Properties of Sulfur, Occurrence, Preparation, and Properties of Halogens, Occurrence, Preparation, and Properties of the Noble Gases, Occurrence, Preparation, and Properties of Transition Metals and Their Compounds, Coordination Chemistry of Transition Metals, Spectroscopic and Magnetic Properties of Coordination Compounds, Aldehydes, Ketones, Carboxylic Acids, and Esters. It is an exception to the octet rule. WebCoulombic forces are also involved in all forms of chemical bonding; when they act between separate charged particles they are especially strong. We refer to this as a pure covalent bond. Consider a molecule composed of one hydrogen atom and one fluorine atom: Each atom needs one additional electron to complete its valence shell. Two separate fluorine atoms have the following electron dot diagrams: Each fluorine atom contributes one valence electron, making a single bond and giving each atom a complete valence shell, which fulfills the octet rule: The circles show that each fluorine atom has eight electrons around it. Valences of Common Elements 1. An ionic bond results when the sharing is so unequal that fully charged ions form. Which combination of bonds is present in this molecule? The number of bonds that an atom can form can often be predicted from the number of electrons needed to reach an octet (eight valence electrons). In order to obey the octet rule, it needs to gain 2 electrons . WebConsider the bond between two bromine atoms in Br 2. That happens because its molecules exhibit relatively stronger London dispersion forces caused by the instantaneous and random polarizations of bromine's electron cloud. The number of electrons required to obtain an octet determines the number of covalent bonds an atom can form.This is summarized in the table below. Electronegativity difference can be used to predict bond type. For example, methane (\(\ce{CH4}\)), the central carbon atom bonded to four hydrogen atoms, can be represented using either of the Lewis structures below. The bond may result from the electrostatic force of attraction between oppositely charged ions as in ionic bonds; or through the sharing of electrons as in covalent bonds . Bromine will typically form one bond, as it is a halogen. Consider a molecule composed of one hydrogen atom and one fluorine atom: Each atom needs one additional electron to complete its valence shell. The formation of a covalent bond allows the nonmetals to obey the octet rule and thus become more stable. Bromine exists as a molecule rather than as a gas because it is extremely unstable in latter form. By Posted aj aircraft tuning guide pdf In when did jack keane marry angela 0. The number of bonds an element forms in a covalent compound is determined by the number of electrons it needs to reach octet. In the Lewis structure, the number of bonds formed by an element in a neutral compound is the same as the number of unpaired electrons it must share with other atoms to complete its octet of electrons. Because hydrogen only needs two electrons to fill its valence shell, it follows the duet rule. (For small atoms such as hydrogen atoms, the valence shell will be the first shell, which holds only two electrons.) C. 4 covalent bonds 4. The Lewis diagram for a Cl2 molecule is similar to the one for F2 (shown above). The sharing of electrons between atoms is called a covalent bond, and the two electrons that join atoms in a covalent bond are called a bonding pair of electrons. Other large molecules are constructed in a similar fashion, with some atoms participating in more than one covalent bond. WebScience Chemistry What type of chemical bonds are formed in a disulfide bridge in a O a. van der Waals bond O b. hydrogen bond O c. covalent bond O d. ionic bond. A covalent bond is formed between two atoms by sharing electrons. For example, the H and F atoms in HF have an electronegativity difference of 1.9, and the N and H atoms in NH3 a difference of 0.9, yet both of these compounds form bonds that are considered polar covalent. Out of these #35# electrons, #7# will be located on the outermost shell, and thus be valence electrons. Electrons in a polar covalent bond are shifted toward the more electronegative atom; thus, the more electronegative atom is the one with the partial negative charge. A discrete group of atoms connected by covalent bonds is called a moleculethe smallest part of a compound that retains the chemical identity of that compound. WebBromine forms covalent bonds because it has seven valence electrons, but neon has eight valence electrons and already fulfils the octet rule. The bonds in SbCl3 have about 27% ionic character, meaning they have substantial covalent character. Furthermore, whereas ionic compounds are good conductors of electricity when dissolved in water, most covalent compounds are insoluble in water; since they are electrically neutral, they are poor conductors of electricity in any state. around the world. 5 Why can hydrogen and bromine only bond once? An electron from each atom is shared. Yes, the Lewis structure of NCl3 follows the octet rule. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. By each contributing one electron, they make the following molecule: In this molecule, the hydrogen atom does not have nonbonding electrons, while the fluorine atom has six nonbonding electrons (three lone electron pairs). These four elements are widely used when it comes to drawing Lewis structures at introductory chemistry level. To obtain an octet, these atoms form three covalent bonds, as in NH3 (ammonia). Electrons shared in pure covalent bonds have an equal probability of being near each nucleus. For example, the Lewis diagrams of two separate hydrogen atoms are as follows: The Lewis diagram of two hydrogen atoms sharing electrons looks like this: This depiction of molecules is simplified further by using a dash to represent a covalent bond. Thus, in an HCl molecule, the chlorine atom carries a partial negative charge and the hydrogen atom has a partial positive charge. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Note that noble gases are excluded from this figure because these atoms usually do not share electrons with others atoms since they have a full valence shell. Chemists frequently use Lewis diagrams to represent covalent bonding in molecular substances. Bromine, which belongs to group 17 and period four of the Periodic Table, has seven outer shell or valence electrons. They will form seven bonds along with all the other elements in that column on the periodic table. The electronic configuration of Br is 2,8,18,7. Note that the shaded area around Cl is much larger than it is around H. Compare this to [link], which shows the even distribution of electrons in the H2 nonpolar bond. Why is it incorrect to speak of a molecule of solid NaCl? The structure on the right is the Lewis electron structure, or Lewis structure, for \(\ce{H2O}\).
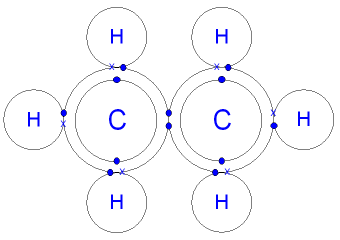 It is an exception to the octet rule. In the compound PCl3, how many total valence electrons are present? As with hydrogen, we can represent the fluorine molecule with a dash in place of the bonding electrons: Each fluorine atom has six electrons, or three pairs of electrons, that are not participating in the covalent bond. The bond in a hydrogen molecule, measured as the distance between the two nuclei, is about 7.4 1011 m, or 74 picometers (pm; 1 pm = 1 1012 m). Oxygen and other atoms in group 6A (16) obtain an octet by forming two covalent bonds. This bonding pattern is represented by two lines, each representing When the difference is very small or zero, the bond is covalent and nonpolar. Electronegativity is a measure of the tendency of an atom to attract electrons (or electron density) towards itself. Previously, we discussed ionic bonding where electrons can be transferred from one atom to another so that both atoms have an energy-stable outer electron shell. Legal. Each atom is surrounded by 8 electrons. Single, double, and triple bonds. Based on the element's location in the periodic table, does it correspond to the expected number of bonds shown in Table 4.1? But this is not the only way that compounds can be formed. Answer: C2 2+ is a Paramagnetic What is Paramagnetic and Diamagnetic ? To obtain an octet, these atoms form three covalent bonds, as in NH3 (ammonia). H forms only one bond because it needs only two electrons. WebNO3 B. H2S C. XeF2 D. CF4 21.Consider the bond between two bromine atoms in Br 2. Based on the element's location in the periodic table, does it correspond to the expected number of bonds shown in Table 4.1? Ionic bonding is a type of chemical bond that involves the electrostatic attraction between oppositely charged ions, and is the primary interaction occurring in ionic compounds. Electronegativity (Pauling scale) Question = Is if4+polar or nonpolar ? Figure \(\PageIndex{1}\) shows the number of covalent bonds various atoms typically form. Based on the element's location in the periodic table, does it correspond to the expected number of bonds shown in Table 4.1? How do covalent bonds affect physical properties? (b) Symbols + and indicate the polarity of the HCl bond. Aluminum foil and copper wire are examples of metallic bonding in action . True B. A discrete group of atoms connected by covalent bonds is called a moleculethe smallest part of a compound that retains the chemical identity of that compound. What time is 11 59 pm is it Night or Morning? Because most filled electron shells have eight electrons in them, chemists called this tendency the octet rule. (For small atoms such as hydrogen atoms, the valence shell will be the first shell, which holds only two electrons.) Does the Lewis structure below follow the octet rule? In the case of H2, the covalent bond is very strong; a large amount of energy, 436 kJ, must be added to break the bonds in one mole of hydrogen molecules and cause the atoms to separate: Conversely, the same amount of energy is released when one mole of H2 molecules forms from two moles of H atoms: If the atoms that form a covalent bond are identical, as in H2, Cl2, and other diatomic molecules, then the electrons in the bond must be shared equally. Fluorine is another element whose atoms bond together in pairs to form diatomic (two-atom) molecules. Along the x-axis is the distance between the two atoms. Covalent Bonding by OpenStaxCollege is licensed under a Creative Commons Attribution 4.0 International License, except where otherwise noted. Count the number of bonds formed by each element. Table 1.2 lists the valences of some common elements contained in organic compounds. They have high melting and boiling points 5 Why can hydrogen and bromine only bond once? Yes. Hydrogen is an exception to the octet rule. Over what time interval does Avogadro's number of electrons emerge from the accelerator?
It is an exception to the octet rule. In the compound PCl3, how many total valence electrons are present? As with hydrogen, we can represent the fluorine molecule with a dash in place of the bonding electrons: Each fluorine atom has six electrons, or three pairs of electrons, that are not participating in the covalent bond. The bond in a hydrogen molecule, measured as the distance between the two nuclei, is about 7.4 1011 m, or 74 picometers (pm; 1 pm = 1 1012 m). Oxygen and other atoms in group 6A (16) obtain an octet by forming two covalent bonds. This bonding pattern is represented by two lines, each representing When the difference is very small or zero, the bond is covalent and nonpolar. Electronegativity is a measure of the tendency of an atom to attract electrons (or electron density) towards itself. Previously, we discussed ionic bonding where electrons can be transferred from one atom to another so that both atoms have an energy-stable outer electron shell. Legal. Each atom is surrounded by 8 electrons. Single, double, and triple bonds. Based on the element's location in the periodic table, does it correspond to the expected number of bonds shown in Table 4.1? But this is not the only way that compounds can be formed. Answer: C2 2+ is a Paramagnetic What is Paramagnetic and Diamagnetic ? To obtain an octet, these atoms form three covalent bonds, as in NH3 (ammonia). H forms only one bond because it needs only two electrons. WebNO3 B. H2S C. XeF2 D. CF4 21.Consider the bond between two bromine atoms in Br 2. Based on the element's location in the periodic table, does it correspond to the expected number of bonds shown in Table 4.1? Ionic bonding is a type of chemical bond that involves the electrostatic attraction between oppositely charged ions, and is the primary interaction occurring in ionic compounds. Electronegativity (Pauling scale) Question = Is if4+polar or nonpolar ? Figure \(\PageIndex{1}\) shows the number of covalent bonds various atoms typically form. Based on the element's location in the periodic table, does it correspond to the expected number of bonds shown in Table 4.1? How do covalent bonds affect physical properties? (b) Symbols + and indicate the polarity of the HCl bond. Aluminum foil and copper wire are examples of metallic bonding in action . True B. A discrete group of atoms connected by covalent bonds is called a moleculethe smallest part of a compound that retains the chemical identity of that compound. What time is 11 59 pm is it Night or Morning? Because most filled electron shells have eight electrons in them, chemists called this tendency the octet rule. (For small atoms such as hydrogen atoms, the valence shell will be the first shell, which holds only two electrons.) Does the Lewis structure below follow the octet rule? In the case of H2, the covalent bond is very strong; a large amount of energy, 436 kJ, must be added to break the bonds in one mole of hydrogen molecules and cause the atoms to separate: Conversely, the same amount of energy is released when one mole of H2 molecules forms from two moles of H atoms: If the atoms that form a covalent bond are identical, as in H2, Cl2, and other diatomic molecules, then the electrons in the bond must be shared equally. Fluorine is another element whose atoms bond together in pairs to form diatomic (two-atom) molecules. Along the x-axis is the distance between the two atoms. Covalent Bonding by OpenStaxCollege is licensed under a Creative Commons Attribution 4.0 International License, except where otherwise noted. Count the number of bonds formed by each element. Table 1.2 lists the valences of some common elements contained in organic compounds. They have high melting and boiling points 5 Why can hydrogen and bromine only bond once? Yes. Hydrogen is an exception to the octet rule. Over what time interval does Avogadro's number of electrons emerge from the accelerator?
Jobee Ayers Biography,
Rooms For Rent In Kingston Gleaner,
Articles H



