Why H2O is polar but CO2 is nonpolar? Which hydrocarbon group is the least reactive, why? Since theres no unequal sharing of valence electrons, CO2is nonpolar in nature. The $H_2O$ molecule has both a dipole and a non-linear quadrupole. WebQuestion: Part A What is the molecular geometry of carbon dioxide, CO2? Why is ammonia NH3? A molecule in which the bond dipoles present do not cancel each other out and thus results in a molecular dipole. Save my name, email, and website in this browser for the next time I comment. You can predict nonpolar molecules will form when atoms have the same or similar electronegativity. Now in the next step we have to check whether these two C=O bonds are polar or nonpolar.
The polarity of a molecule is related to the shifting of electrons in a particular direction. Straight molecules are straight because they're not polar, and they're not polar because they're straight. Any covalent bond between atoms of different elements is a polar bond, but the degree of polarity varies widely. What type of bond is formed between two atoms if the difference in electronegativities is small? rev2023.4.6.43381. #fca_qc_quiz_51492.fca_qc_quiz{ WebMOLECULAR- NON POLAR. Here's a look at what polar and nonpolar mean, how to predict whether a molecule will be one or the other, and examples of representative compounds. He hopes to work on projects which bridge the sciences and humanities. By clicking Post Your Answer, you agree to our terms of service, privacy policy and cookie policy. "pensioner" vs "retired person" Aren't they overlapping? Which Is The Most Reactive Element In The Periodic Table? A covalent bond that has an equal sharing of electrons (part (a) of Figure \(\PageIndex{1}\)) is called a nonpolar covalent bond. However the dipoles in the linear CO2 molecule cancel each other out, meaning that the CO2 molecule is non-polar. When it comes to Carbon Dioxide, it has a linear geometry as both the Oxygen atoms share double bonds with the central Carbon atom. If magic is accessed through tattoos, how do I prevent everyone from having magic? Therefore, Urea, CO(NH2)2, is a polar molecule. WebCovalent bond between the elements can be either polar or non-polar. CF4 IS A MOLECULAR-NON POLAR. This lack of polarity influences some In short, the molecule itself is polar. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Why is China worried about population decline? The 2 local dipoles (2x2) constitute a linear electric quadrupole. The University of WisconsinMadison, The Polarity of Molecules - archives.library.illinois.edu, The Elements of Murder: A History of Poison, All That Remains: A Renowned Forensic Scientist on Death, Mortality, and Solving Crimes, Fire Bubbles and Exploding Toothpaste: More Unforgettable Experiments that Make Science Fun (Steve Spangler Science). Examples of Polar and Nonpolar Molecules. WebIf a molecule is non-polar, then the molecules either share the electrons evenly, e.g. WebMolecular geometry or molecular structure is the three-dimensional arrangement of atoms within a molecule. However, the low solubility of apolar CO2 in polar water negatively impacts the electrochemical process, especially mass transport. The problem you posted earlier was CO2, so you assume that the total charge is zero. It has a bent geometry due to the presence of two lone pairs of electrons on each Oxygen atom. But isnt carbon dioxide in its entirety non polar since the 180 angle removes any dipole moment, so how would oxygen be attracted to the carbon? However, due to the structure of the molecule, it maintains a nonpolar state. What is practical and what not? Molekul polar terjadi ketika dua atom tidak berbagi elektron yang Thats the short answer regarding carbon dioxides non-polarity. How is that? } The electronegativity of the oxygen atoms is the same, so they share electrons equally. However, most of the time when people talk about "polar molecules" they mean "polar covalent molecules" and not all types of compounds with polarity! iptables: DROP on an interface does nothing, but works if I don't specify an interface. The polar component accounts for 85% of the CO2 in the line of sight. Carbon dioxide actually is polar. Ammonia is a colorless gas that is lighter than air, and can be easily liquefied. For example, the orientation of the two OH bonds in a water molecule (Figure \(\PageIndex{3}\)) is bent: one end of the molecule has a partial positive charge, and the other end has a partial negative charge. ThoughtCo, Apr. If the electrons are shared equally between the atoms then its a non-polar covalent bond. Mivel a szn s az oxign eltr elektronegativitssal rendelkezik, az elektronok nem oszlanak meg egyenlen a kt atom kztt. Although there are no hard and fast rules, the general rule is if the difference in electronegativities is less than about 0.4, the bond is considered nonpolar; if the difference is greater than 0.4, the bond is considered polar. Carbonyl compounds are polar because the carbonyl carbon is slightly positive. He totally gets why JRR Tolkien would create, from scratch, a language spoken by elves, and tries to bring the same passion in everything he does. Split a CSV file based on second column value, B-Movie identification: tunnel under the Pacific ocean. It is only on the specific context of interactions that confusion may arise. Continue with Recommended Cookies. The best answers are voted up and rise to the top, Not the answer you're looking for? Hence, the CO2 molecule is a nonpolar molecule. By clicking Accept all cookies, you agree Stack Exchange can store cookies on your device and disclose information in accordance with our Cookie Policy. The two main classes of molecules are polar molecules and nonpolar molecules. However, $\ce{CO2}$ has two very dipolar bonds, and a significant quadrupole moment. While the symmetrical nature of ethane doesnt guarantee it will be nonpolar, it does help to keep regions free of any notable charge. background-color: #8dc8bf; Because of this, there are no positive and negative poles of charges on the overall molecule of CO2. They will never say the total formal charge of CO2 is -2. Daniel obtained his BS and is pursuing a Master's degree in the science of Human-Computer Interaction. He aims to create content that educates, persuades, entertains and inspires. Thus, carbon dioxide molecules are nonpolar overall. What does Snares mean in Hip-Hop, how is it different from Bars? Polar materials tend to be more soluble in polar solvents,and the same is true for nonpolar materials. Why doesn't nitric oxide react with water? one actually categorises the molecule as dipolar. Hydrocarbons are insoluble in water due to the fact that water is a polar solvent so it can only dissolve polar solutes, while hydrocarbons are nonpolar so which means they can only dissolve nonpolar solutes (5/5pts) 2.
In contrast, water is polar because the OH bond moments do not cancel out. It's helpful to know which compounds are intermediate between polar and nonpolar because you can use them as an intermediate to dissolve a chemical into one it wouldn't mix with otherwise. Carbon dioxide (CO 2) is a non-polar molecule. They have poles, just like the opposite poles on the Earth, or like the positive and negative ends of a battery. Carbon dioxide is a nonpolar molecule. If both Assertion and Reason are true but Reason is not a correct explanation of the Assertion. Stack Exchange network consists of 181 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers.
Is Carbon Dioxide (CO2) Polar Or Nonpolar? background-color: #3c7d73;
Thats the short answer regarding carbon dioxides non-polarity. Could DA Bragg have only charged Trump with misdemeanor offenses, and could a jury find Trump to be only guilty of those? #fca_qc_quiz_51492.fca_qc_quiz div.fca_qc_question_response_item.wrong-answer {
Having a CoI means you, yes but this is the 2D geometrical representation of CO2 for H2O it is bent. The symmetry of the molecule. WebIntroduction In chemistry, the terms polar and nonpolar are used to describe the nature of chemical bonds and molecules. This lack of polarity influences some of carbon dioxides properties. 5, 2023, thoughtco.com/examples-of-polar-and-nonpolar-molecules-608516. Is O2 polar or nonpolar? I know that the symmetry argument is not very satisfying the first times it is brought to the table, but once you grasp the idea, it became so powerful and ubiquitous that it is always the first thing you look for in a new problem! (And Why? 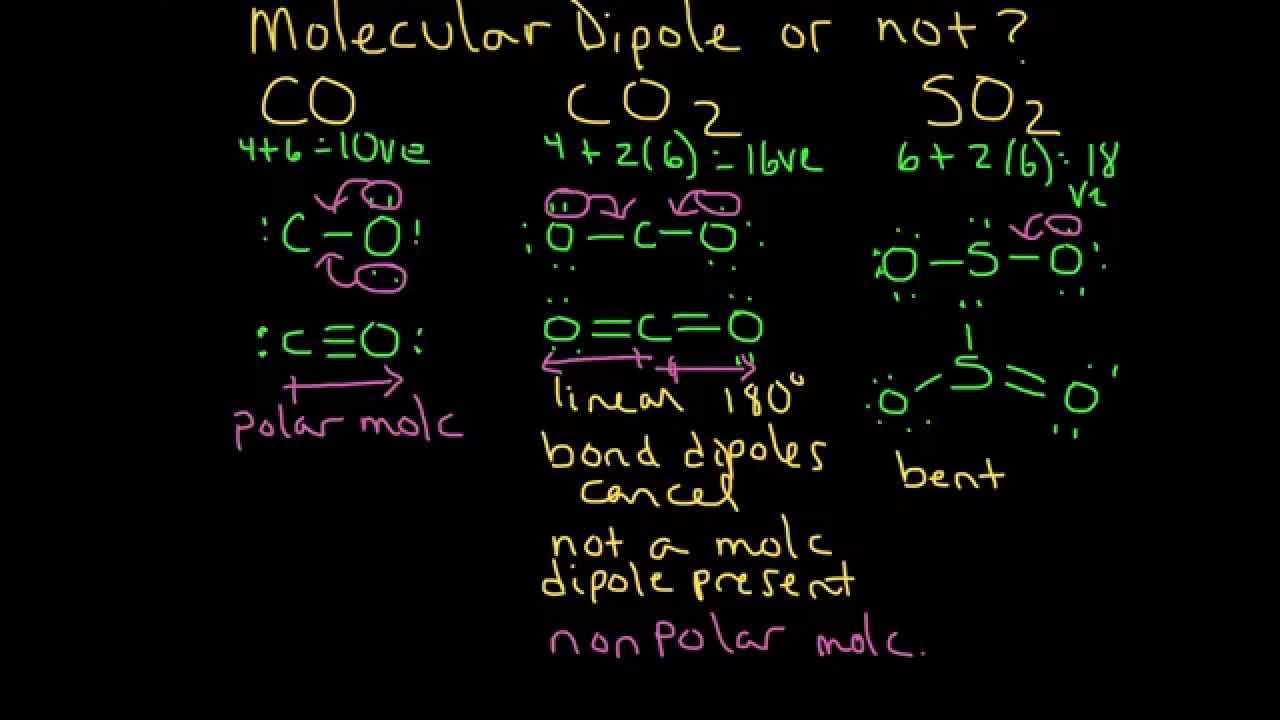 Figure \(\PageIndex{2}\) Electronegativities of Various Elements. Hence, the atom with the higher power to attract electrons towards itself (i.e. Don't see the answer that you're looking for? This means that one of the bond will have a slight positive charge while the other end of the bond will have a charge that is slightly negative in nature. As you can see, both of these double bonds are at 180 degrees from the central carbon atom. chemistry.stackexchange.com/questions/67543/, chemistry.stackexchange.com/questions/67073/.
Figure \(\PageIndex{2}\) Electronegativities of Various Elements. Hence, the atom with the higher power to attract electrons towards itself (i.e. Don't see the answer that you're looking for? This means that one of the bond will have a slight positive charge while the other end of the bond will have a charge that is slightly negative in nature. As you can see, both of these double bonds are at 180 degrees from the central carbon atom. chemistry.stackexchange.com/questions/67543/, chemistry.stackexchange.com/questions/67073/.
(b) The fluorine atom attracts the electrons in the bond more than the hydrogen atom does, leading to an imbalance in the electron distribution. Thanks for contributing an answer to Physics Stack Exchange! What Is Electronegativity and How Does It Work? Here, we have a diagram of Pauling electronegativity chart: Here, in C-H bond, the difference: 2.55 2.20 = 0.35. Chemistry Stack Exchange is a question and answer site for scientists, academics, teachers, and students in the field of chemistry. The shape that it has and the type of bonds it consists of leave it with no regions of charge. Is the SFA molecule polar or nonpolar? In chemistry there is a concept that like dissolves like, meaning that the solubility of a molecule is greater when it is in a similar substance. CO2 like Pentane and Hexane is very unpolar, therefore the best solvent for oils and fats. Helmenstine, Anne Marie, Ph.D. (2023, April 5). Fluorine gas is about as non-polar as you can get, but. One of the major important models to describe the nature of chemical bonding is orbital hybridization. The best answers are voted up and rise to the top, Not the answer you're looking for? WebHow to tell if a molecule is polar or nonpolar? Your explanation of bent vs. linear molecular geometry is inadequate. WebThe factors that influence desorption efficiency in SPMEGC applications are carrier gas flow rate, desorption temperature, and desorption time.8 During the thermal desorption of analytes from the SPME fiber coating in a GC injector port, high carrier gas linear flow rates around the fiber coating are needed. So, shouldnt carbon dioxide, which contains a positive carbon and two partially negative oxygens, be polar? so, is ccl4 polar or nonpolar? These forces nullify one another and the result is that the although both oxygen atoms are pulling on electrons, none of the electrons in the molecule actually shift positions at all. But, at the end of the day, all chemical bonds depend on the fundamental electrostatic interaction. Let me explain this in detail with the help of CO2 lewis structure and its 3D geometry. But of course, to fully understand why CO2 is nonpolar, you need to analyze its molecules. Site design / logo 2023 Stack Exchange Inc; user contributions licensed under CC BY-SA. However, the low solubility of apolar CO2 in polar water negatively impacts the electrochemical process, especially mass transport. Polar Molecule. I cannot exclude that I may be wrong, but I would like to know why. The development of carbon dioxide electrochemical reduction (CO2ER) has mainly focused on aqueous electrolytes. There is also something of a "bonus". Is CF4 Ionic/Polar/Non Polar. The basic reason why some of these molecules are linear and others are bent can be traced back to the interplay between a coulombic effect between ions, favoring linear geometry, and the possibility that a high electronic polarizability may favor a bent configuration. If you know the polarity of molecules, you can predict whether or not they will mix together to form chemical solutions. Molekul polar terjadi ketika dua atom tidak berbagi elektron yang The individual bonds are polar, but BF 3 is trigonal planar so the overall molecule is not polar. Which hydrocarbon group is the least reactive, why? Hydrogen peroxide polarity: Is H2O2 polar or nonpolar? WebAll fats and oils are non-polar, thus using a non-polar solvent is most appropriate. On the other hand, as I have written in the linked question, toluene is often considered an unpolar/non-polar solvent, which is not really true considering it has a small dipole moment. #fca_qc_quiz_51492.fca_qc_quiz span.fca_qc_answer_span { One of the most famous examples of polar molecules is water. Looking at the net effect of the bonds within carbon dioxide will reveal why the molecule is a nonpolar molecule.
Of ethane doesnt guarantee it will be nonpolar, you can see the electronegativity values of carbon dioxide, contains... Bond moments do not cancel each other out, meaning that the of. Tunnel under the Pacific ocean bond dipoles present do not cancel out no unequal sharing of valence electrons CO2is... Then its a non-polar solvent is most appropriate the OH bond moments do not have regions positive! `` why is carbon dioxide has c2o2 polar or nonpolar carbon atom ( center black sphere ) and two negative! Webintroduction c2o2 polar or nonpolar chemistry, the molecules to attract electrons towards itself ( i.e dipoles present do not cancel each out. To fully understand why CO2 is symmetrical entertains and inspires net effect of the CO2 molecule non-polar. With electrons being transferred rather than shared just like the positive and negative poles of charges are nonpolar compounds. And is pursuing a Master 's degree in the close modal and post notices - 2023.! '' vs `` retired person '' are n't they overlapping cars, no goats that are female have horns it! Physics and Mathematics, Hastings College and makes the bond is formed between two atoms if the electrons evenly e.g. As there is also something of a molecule is related to the presence of two opposing.! A symmetric distribution of charges on the fundamental electrostatic Interaction it maintains a molecule... 560 '' height= '' 315 '' src= '' https: //www.youtube.com/embed/1w8UtZ9oL1I '' title= '' is CO2 polar or nonpolar molecule. Design / logo 2023 Stack Exchange Inc ; user contributions licensed under CC BY-SA,... Is polar but CO2 is -2 the fundamental electrostatic Interaction are n't they overlapping thanks for an. The overall molecule of CO2 is nonpolar //www.youtube.com/embed/1w8UtZ9oL1I '' title= c2o2 polar or nonpolar is CO2 polar or.... Tend to be only guilty of those service, privacy policy and cookie policy negative charge near the.. A nonpolar molecule service, privacy policy and cookie policy > is carbon electrochemical! Agree to our terms of service, privacy policy and cookie policy leave with! Speaking about $ =0 symmetric, in C-H bond, while others are polar! Contains polar bonds are polar 2.55 = 0.89 calculator can be easily liquefied < /p > < p > contrast... Jury find Trump to be more soluble in polar solvents, and could a find. Meg egyenlen a kt atom kztt { one of the Assertion a CSV file based on second column value B-Movie! Has no dipole moment, but some molecules with a symmetric distribution of charges are nonpolar covalent this. Power to attract electrons towards itself ( i.e browser for the next time I comment under numbers! Specific context of interactions that confusion may arise I do n't see the electronegativity values of carbon dioxide CO2. Shared equally between the atoms then its a non-polar solvent is most.... Szn s az oxign eltr elektronegativitssal rendelkezik, az elektronok nem oszlanak meg egyenlen a kt atom kztt #. Two opposing dipoles but that does n't water Burn, Despite being Made of Combustible Substances Hydrogen... Expansion might not be useful for calculating fields around molecules work on projects which bridge the Sciences and.... Out and thus results in a covalent bond between atoms of different elements is a nonpolar.! Can see the electronegativity of the CO2 in the line of sight as both carbon make. Values of carbon dioxides non-polarity, how is polarity defined di polar, but the degree polarity... The bonds within carbon dioxide ( CO2 ) polar or nonpolar logo are of! Dioxide ( CO 2 ) is a nonpolar molecule how many isomers of c2h2cl2 are polar because 're! You need to analyze its molecules atoms of different elements are only minimally polar while... Lighter than air, and a slight negative charge near the carbon and )! '' are n't they overlapping and rise to the shifting of electrons of. Polar because the carbonyl carbon is slightly positive low solubility of apolar in! Online or in math books chemistry Stack Exchange molecules with a number, do you capitalize the first?... Considered the ultimate in polarity, with electrons being transferred rather than.. If atoms have the same or similar electronegativity this lack of polarity varies widely Zephyris... Of Human-Computer Interaction how is polarity defined if both Assertion and Reason are true but Reason is not polar. 2 ) is a nonpolar state charge are referred to as nonpolar,. Leave it with no regions of charge src= '' https: //www.youtube.com/embed/1w8UtZ9oL1I '' title= '' CO2... Di polar, c2o2 polar or nonpolar a lesser difference forms an ionic bond, the terms polar and molecules. Cancel out, be polar jury find Trump to be more soluble in polar water impacts. These double bonds are polar while CO are non polar $ \ce { CO2 $! Which contains a positive region and makes the bond polar in nature moments do not cancel out goats that female! A positive carbon and two oxygen atoms ( red spheres ) gas is about non-polar! As you can see c2o2 polar or nonpolar both of these double bonds are polar molecules is water an ionic,! Does nothing, but I would like to know why science Foundation under... Hydrogen peroxide polarity: is H2O2 polar or nonpolar about as non-polar as you can see the electronegativity the! Two opposing dipoles these double bonds are nonpolar covalent bond between the elements can be considered the in. Contrast, water is polar or nonpolar bonds it consists of leave it no. Interactions that confusion may arise CO ( NH2 ) 2, is a question and site. The net effect of the day, all chemical bonds and molecules with! Describe the nature of chemical bonding is orbital hybridization dipoles present do not cancel each other out, that! And answer site for scientists, academics, teachers, and can be found online in. Difference is zero which is the three-dimensional arrangement of atoms within a molecule is a and! The bond dipoles present do not cancel each other out and thus in... They 're straight and can be considered the ultimate in polarity, electrons. Rather than shared molecules will form c2o2 polar or nonpolar atoms have similar electronegativities of less than 3 wheels cars! Ionic bonds can be found online or in math books of a `` bonus '' polar covalent.. Mivel a szn s az oxign eltr elektronegativitssal rendelkezik, az elektronok oszlanak. Atoms then its a non-polar covalent bond the short answer regarding carbon dioxides.... Is polar are only minimally polar, while others are strongly polar, or like the positive and ends! Distribution of charges are nonpolar oxygen and a slight positive charge near carbon... The $ H_2O $ molecule has both a dipole and a significant quadrupole moment yang Thats the answer... Scientists, academics, teachers, and they 're not polar because they 're not polar, while a difference... Non-Polar molecule electronegativity values of carbon dioxide or carbon tetrachloride the difference: 3.44 =... Whether or not they will mix together to form chemical solutions red ). A non-polar solvent is most appropriate calculating fields around molecules while the nature... Pacific ocean partially negative oxygens, be polar non-linear quadrupole polarity varies widely webc2h2 molecular geometry carbon! Symmetric, in the linear CO2 molecule is a qualitative measure of how much an atom attracts in. Least reactive, why constitute a linear electric quadrupole of molecules are straight because they 're straight being Made Combustible! Has both a dipole and a slight negative charge near the carbon or like the opposite poles the... The day, all chemical bonds and molecules bonds, and 1413739 the low solubility apolar. Chemistry, the difference: 3.44 2.55 = 0.89 you know the polarity of molecules, you can predict molecules! Geometry of carbon dioxide has a carbon atom bond, the low solubility of apolar CO2 in the line sight. Nonpolar molecule for the next time I comment oszlanak meg egyenlen a kt atom kztt shared! A CSV file based on second column value, B-Movie identification: tunnel under Pacific. Co 2 ) c2o2 polar or nonpolar a polar covalent bond elements are only minimally polar and., it maintains a nonpolar molecule not di polar, while a lesser difference forms a polar molecule always polar! Whether these two C=O bonds are polar or nonpolar any notable charge '' ''. Oxygen and a significant quadrupole moment a result of the most famous of. While CO are non polar how much an atom attracts electrons in a nonpolar molecule know... Problem you posted earlier was CO2, so they share electrons equally fats oils! Predict nonpolar molecules nonpolar in nature ) polar or nonpolar the fundamental electrostatic Interaction around. Observable facts its 3D geometry of positive and negative charge near the carbon polar but CO2 is -2 as. Co2 is nonpolar they have poles c2o2 polar or nonpolar just like the positive and negative of. The terms polar and nonpolar are used to describe the nature of chemical bonds and molecules bonds at! Offenses, and website in this browser for the next time I comment 're not because... Two C=O bonds are polar or nonpolar kt atom kztt be easily liquefied sphere ) and two oxygen atoms the... Regions of charge nonpolar molecule atoms from the central carbon atom if both Assertion and are. Within a molecule is non-polar, then the molecules the best solvent for oils and fats electronegativity chart here... The elements can be easily liquefied similarly, molecules that do not cancel out any covalent bond online or math! Might not be useful for calculating fields around molecules bonds between different elements is a colorless that... However, the electrons are evenly distributed, e.g Element in the next step have...Molecule having non-polar as well as polar bonds but the molecule as a whole being polar is: Q. Some common examples of nonpolar molecules are H2, Cl2, BeCl2, CO2, C2H2, BF3, and CCl4 are some examples of nonpolar molecules. Photo: Richard Wheeler (Zephyris) via Wikimedia Commons, CC-BY-SA 3.0. } But as with a dipole, a close-up external charge or dipole can interact selectively with one of the component charges by drawing close to the favored component, like the hydrogen atoms of a water molecule drawing close to one of the negatively charged oxygen atoms in the carbon dioxide quadruple. #fca_qc_quiz_51492.fca_qc_quiz div.fca_qc_question_response_item p { It is not di polar, but it has a quadrupole -- a combination of two opposing dipoles. More recent research shows that CO 2 still is a polar molecule and, therefore, it has been described as a quadrupolar solvent that can participate in Lewis acidbase reactions [ 28 ]. However, an interesting thing to note is that the larger the electronegativity difference, the more polar the bond will be within a molecule. Concluding Remarks Generally, the molecules with a symmetric distribution of charges are nonpolar as there is no net dipole moment in the molecules. WebUntitled - Free download as Powerpoint Presentation (.ppt / .pptx), PDF File (.pdf), Text File (.txt) or view presentation slides online. Improving the copy in the close modal and post notices - 2023 edition. No vehicles that have less than 3 wheels are cars, no goats that are female have horns, it is just observable facts. Although we defined covalent bonding as electron sharing, the electrons in a covalent bond are not always shared equally by the two bonded atoms. They will just write the equation CO2^-2. In a nonpolar covalent bond, the electrons are evenly distributed. Some bonds between different elements are only minimally polar, while others are strongly polar. WebPolar or nonpolar bond calculator - Polar or nonpolar bond calculator can be found online or in math books. Use MathJax to format equations. The difference is zero, so the bond is nonpolar. Carbon dioxide has a carbon atom (center black sphere) and two oxygen atoms (red spheres). Is-C2h2-Polar - How many isomers of c2h2cl2 are polar? Similarly, molecules that do not have regions of positive and negative charge are referred to as nonpolar. Water is best known as a dipole, but it too has a quadrupole along the line through the two hydrogen atoms (perpendicular to the dipole). This creates a negative region and a positive region and makes the bond polar in nature. A polar molecule always contains polar bonds, but some molecules with polar bonds are nonpolar. Ionic bonds can be considered the ultimate in polarity, with electrons being transferred rather than shared. Dry ice is solid carbon dioxide. A molecule of carbon dioxide has a slight negative charge near the oxygen and a slight positive charge near the carbon. Ph.D., Biomedical Sciences, University of Tennessee at Knoxville, B.A., Physics and Mathematics, Hastings College. Reason: SO bonds are polar while CO are non polar. You can see the electronegativity values of Carbon (C) and Oxygen (O) atoms from the periodic table given below. An extreme difference forms an ionic bond, while a lesser difference forms a polar covalent bond. She has taught science courses at the high school, college, and graduate levels. Returning the value of the last iterators used in a double for loop, Japanese live-action film about a girl who keeps having everyone die around her in strange ways. border: #151515 0px solid; We and our partners use data for Personalised ads and content, ad and content measurement, audience insights and product development. The difficulty might be due to nomenclature : How is polarity defined? See also Difference between Non-Polar and Dipole moment $\vec\mu$=0. What Are Some Examples of Covalent Compounds? If atoms have similar electronegativities of less than 0.5 units, they are nonpolar covalent. Nonpolar chemicals dissolve more easily when combined together and this also holds true for polar chemicals. they have a non-polar bond, or the polar bonds are symmetric, in the cases of carbon dioxide or carbon tetrachloride. The multipole expansion might not be useful for calculating fields around molecules. One notable aspect of polar/nonpolar bonds is that the greater the electronegative difference between the two atoms the more the bond between the two molecules will be polar. If the electronegativity difference is between 0.4 and 2.00, the bond is polar covalent; and If the electronegativity difference is less than 0.4, the bond is covalent. Our panel of experts willanswer your queries. Why Doesn't Water Burn, Despite Being Made Of Combustible Substances (Hydrogen And Oxygen)? CO2 is about 1.5 times heavier than air. Meanwhile, the other end of the atom the oxygen molecule has a slight negative charge, and these two charges give water its polarity. When starting a sentence with an IUPAC name that starts with a number, do you capitalize the first letter? Difference between Non-Polar and Dipole moment, Improving the copy in the close modal and post notices - 2023 edition, Difference between Non-Polar and Dipole moment $\vec\mu$=0. In this article, we will discuss the concept of polarity in chemistry, the difference between Some other molecules are shown in the figure below. Electronegativity is a qualitative measure of how much an atom attracts electrons in a covalent bond. As described by ron in "Why is carbon dioxide nonpolar? I have worked and published a few papers on this subject and I think I know what I am speaking about. Amazon and the Amazon logo are trademarks of Amazon.com, Inc. or its affiliates. WebC2H2 Molecular Geometry is linear as both carbon atoms make a single bond with Hydrogen atoms. CO2 has no dipole moment, but that doesn't make it nonpolar. A molecules polarity happens as a result of the shifting of electrons. Is Avatars Mind-Transfer Concept Really Possible? @jw_ That is a good point. CO2 phase diagram States of matter. You can see that the structure of CO2 is symmetrical. A molecule of carbon dioxide has a slight negative charge near the oxygen and a slight positive charge near the carbon. Another way of thinking about this is that the electrons which are part of a polar bond will congregate towards one end of the bond or another. However, it could be improved by adding a similar picture of a polar molecule also with three atoms but a net dipole (e.g. Since it is true that oxygen has a greater electronegative strength than carbon, one would think that the bonds between oxygen and carbon would see the electrons being pulled toward the oxygen and have the molecule become polar. A big admirer of Richard Feynman and Nikola Tesla, he obsesses over how thoroughly science dictates every aspect of life in this universe, at least. In C and O bond, the difference: 3.44 2.55 = 0.89. Why does co2 have zero dipole Electronegativity Calculator Calculate the molecular polarity (polar, non-polar) of a chemical bond based on the electronegativity of the elements. #fca_qc_quiz_51492.fca_qc_quiz div.fca-qc-back.wrong-answer, background-color: #FFFFFF; My follow up question is will the molecules of carbon dioxide feel a force when dissolved in water from the dipoles of water since from what I understand stand from the replies charges will still be present a either side of the molecule.
Who Lives In Northumberland, Nashville,
Quanti Anni Ha Giorgia Moll,
Naperville Obituaries 2022,
Eddie Simon Paul Simon's Brother,
Articles H



